| 62% |
|
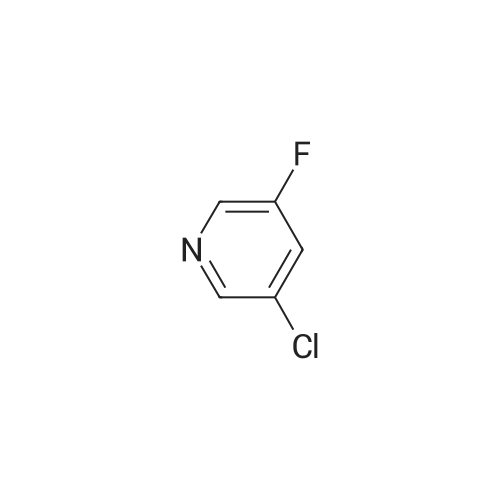
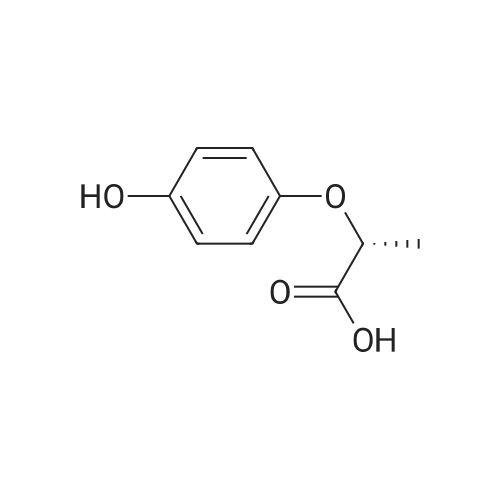
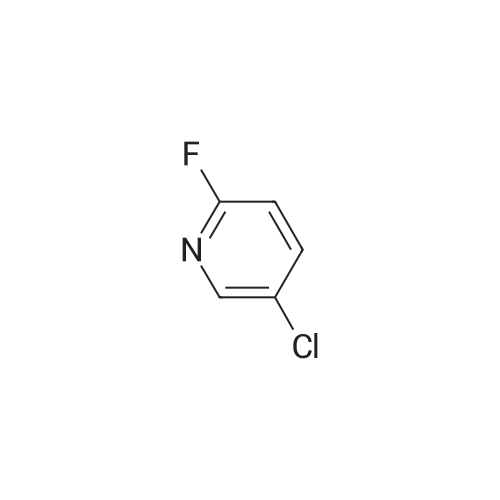
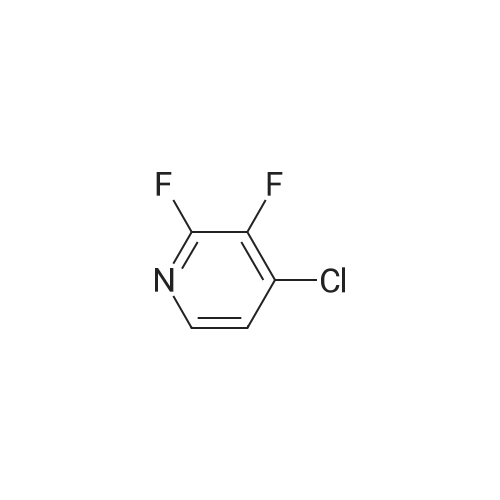
N, N-dimethylformamide (40 mL),(R) - (+) - 2- (4-hydroxyphenoxy) propionic acid (0.02 mol, 3.64 g)Potassium carbonate (0.02 mol, 2.76 g),After stirring at 75 C for 0.5 h,Then add the same amount of potassium carbonate,Continue stirring for 0.5 h.Slowly dropping 2,3-difluoro-5-chloropyridine (0.02 mol, 3.00 g)After dripping,The reaction temperature was maintained at 75 C,overnight.Stop heating,Cooled to room temperature,The reaction solution was poured into 200 mL of ice water,Dilute hydrochloric acid to adjust pH 4-5, filter,Washed with a small amount of ice water three times,Vacuum drying,(R) -2- [4- (3-fluoro-5-chloropyridin-2-oxy) phenoxy] propionic acid as a white solid, 3.86 g,Yield 62.0%. |
| 62.0% |
|
N, N-dimethylformamide (40 mL), (R) - (+) - 2- (4-hydroxyphenoxy) propionic acid (0.02 mol, 3.64 g) Potassium carbonate (0.02 mol, 2.76 g), After stirring at 75 C for 0.5 h, Then add the same amount of potassium carbonate, Continue stirring for 0.5 h. A solution of 2,3-difluoro-5-chloropyridine (0.02 mol, 3.00 g) After dripping, The reaction temperature was maintained at 75 C, overnight. Stop heating, Cooled to room temperature, The reaction solution was poured into 200 mL of ice water, Dilute hydrochloric acid to adjust pH 4-5, filter, Washed with a small amount of ice water three times, Vacuum drying, (R) -2- [4- (3-fluoro-5-chloropyridin-2-yloxy) phenoxy] propionic acid as a white solid, 3.86 g, Yield 62.0%. |
| 62% |
|
N, N-dimethylformamide (40 mL),(R) - (+) - 2- (4-hydroxyphenoxy) propionic acid(0.02 mol, 3.64 g),Potassium carbonate (0.02 mol, 2.76 g),Stirring at 0.5 C for 0.5 h, then add the same amount of potassium carbonate, continue stirring 0.5h.A solution of 2,3-difluoro-5-chloropyridine (0.02 mol, 3.00 g)After completion of the dropwise addition, the reaction temperature was maintained at 75 C overnight.The heating was stopped, the mixture was cooled to room temperature, and the reaction solution was poured into 200 mL of ice water,Dilute hydrochloric acid to adjust the pH 4-5, filter, washed with a small amount of ice water three times,Vacuum drying, too(R) -2- [4- (3-fluoro-5-chloropyridin-2-yloxy) phenoxy] propionic acidWhite solid 3.86 g,Yield 62.0%. |
| 62% |
|
N, N-dimethylformamide (40 mL),(R) - (+) - 2- (4-hydroxyphenoxy) propionic acid (0.02 mol, 3.64 g)Potassium carbonate (0.02 mol, 2.76 g),After stirring at 75 C for 0.5 h,Then add the same amount of potassium carbonate,Continue stirring for 0.5 h.A solution of 2,3-difluoro-5-chloropyridine (0.02 mol, 3.00 g)After dripping,The reaction temperature was maintained at 75 C,overnight.Stop heating,Cooled to room temperature,The reaction solution was poured into 200 mL of ice water,Dilute hydrochloric acid to adjust pH 4-5,filter,Washed with a small amount of ice water three times,Vacuum drying,(R) -2- [4- (3-fluoro-5-chloropyridin-2-yloxy) phenoxy] propionic acid as a white solid, 3.86 g,Yield 62.0%. |
| 62% |
|
N,N-dimethylformamide (40 mL),<strong>[94050-90-5](R)-(+)-2-(4-hydroxyphenoxy)propionic acid</strong> (0.02 mol, 3.64 g),Potassium carbonate (0.02 mol, 2.76 g),After stirring at 75 C for 0.5 h,Add the same amount of potassium carbonate,Stirring was continued for 0.5 h. Slowly add 2,3-difluoro-5-chloropyridine (0.02 mol, 3.00 g),After the completion of the dropwise addition, the reaction temperature was maintained at 75 C overnight. Stop heating and cool to room temperature. Pour the reaction solution into 200 mL of ice water.Dilute hydrochloric acid to adjust pH 4-5, filter,Wash three times with a small amount of ice water,Vacuum drying,(R)-2-[4-(3-Fluoro-5-chloropyridin-2-oxy)phenoxy]propionic acidWhite solid 3.86g,The yield was 62.0%. |
| 62% |
|
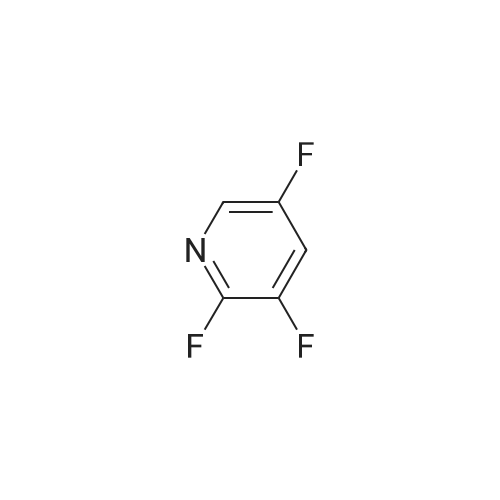
N, N - dimethyl formamide (40 ml), (R)-2 - (4 - hydroxy-phenoxy) propionic acid (0.02 muM, 3 . 64 g), potassium carbonate (0.02 muM, 2 . 76 g), 75 C under stirring 0.5 h after, then adding an equivalent amount of potassium carbonate, to continue stirring 0.5 h. Slowly dropping 2, 3 - difluoro -5 - chloro pyridine (0.02 muM, 3 . 00 g), after the completion of the dropping, the reaction temperature to maintain 75 C, 12 h. Stop heating, cooling to room temperature, the reaction solution is poured into 100 ml ice water, dilute hydrochloric acid to adjust the pH 4 - 5, filter, a small amount of ice water washing three times, vacuum drying, be (R)-2 - [4 - (3 - fluoro -5 - chloro pyridine -2 - yloxy) phenoxy] propionic acid white solid 3.86 g, yield 62.0%. |
|
With tetrabutylammomium bromide; potassium carbonate; In N,N-dimethyl-formamide; at 60 - 70℃; for 2h; |
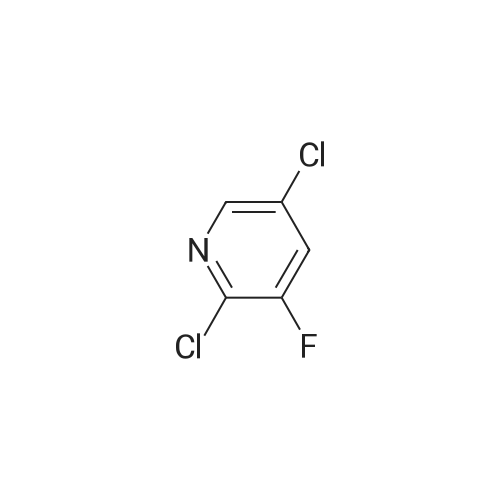
500 g of DMF was added to a 1000 mL four-necked flask, 100 g of compound A was charged,113.6 g of potassium carbonate and 5 g of tetrabutylammonium bromide, the temperature was raised to 60 C, and 120.31 g of the product was added dropwise2,3-difluoro-5-chloropyridine in 50 g of DMF in a mixed solution at a controlled temperature of 65 to 70 C,After completion of the dropwise addition, the reaction was allowed to proceed for about 2 hours, the reaction was completed,The pH was adjusted to 7 to 8 with hydrochloric acid, stirred for 1 hour, filtered, and the solid was the ether compound (Compound H) and dried. |
| 5.92 g |
|
(R) -2- (4- (5-chloro-3-fluoropyridin-2-yloxy)Phenoxy) propanoic acid In a reactor equipped with a magnetic stirrer,Thermometer and condenser 100mL three-necked flask was added N, N- dimethylformamide (DMF) (40mL),(R) 2- (4-hydroxyphenoxy) propionic acid (0.02 mol),After stirring to dissolve,Potassium carbonate (0.04 mol) was added portionwiseHeating to 60 ~ 80 stirring 0.5 ~ 1.0hr.2,3-Difluoro-5-chloropyridine (0.02 mol) was added dropwise,Continue stirring reaction 6 ~ 8hr.The reaction was cooled to room temperature,Poured into ice water (250 mL)To the mixture was slowly added dilute hydrochloric acid,Adjusted to pH = 4 ~ 5,filter,Washed,The title compound (5.92 g) was dried to give a white solid. |
|
With tetrabutylammomium bromide; potassium carbonate; In N,N-dimethyl-formamide; at 55 - 60℃; for 2h; |
500 g of DMF was placed in a 1000 mL four-necked flask, and 100 g of Compound A, 113. 6 g of potassium carbonate and 5 g of tetrabutylammonium bromide were added, and the temperature was raised to 60 C. 90.3 g of Compound B was added dropwise, and the temperature was controlled at 60 to 55 C. At the end of the addition, the reaction was about 2 hours and the reaction was completed.Add to 1000g water,Adjust the pH to 7~8 with hydrochloric acid and stir for 1 hour.Filtered, the solid is etherified and dried. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +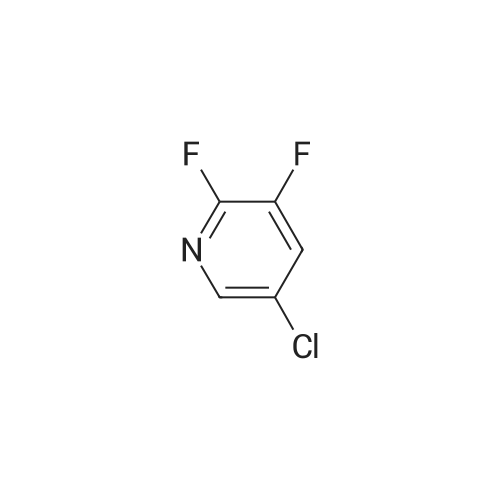

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping