| 83% |
|
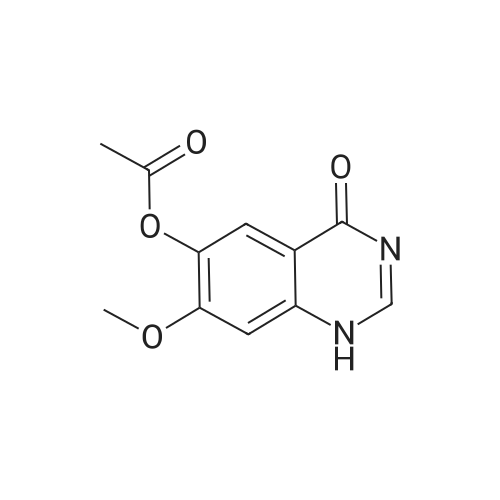
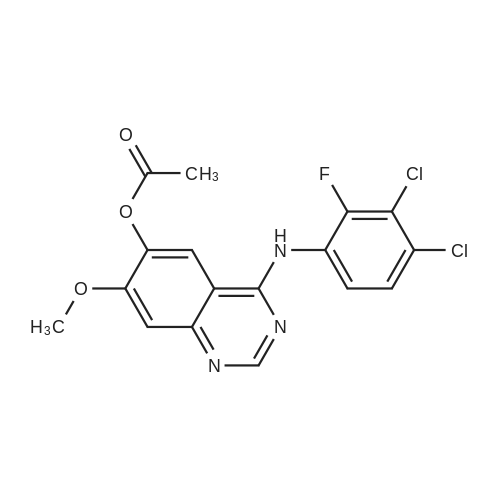
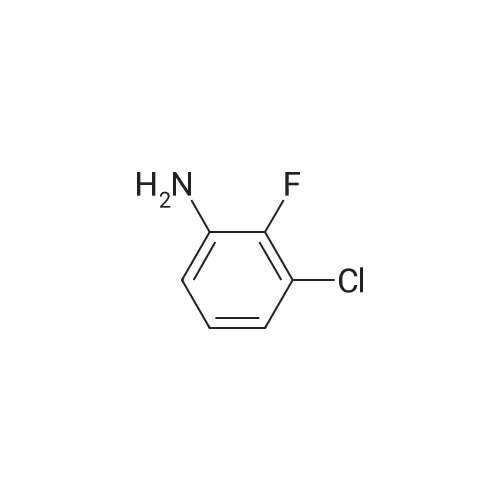
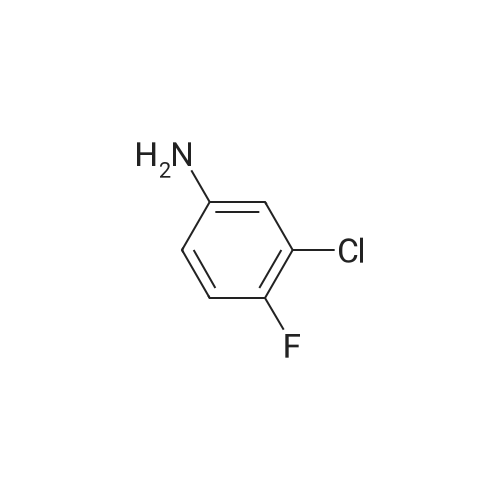
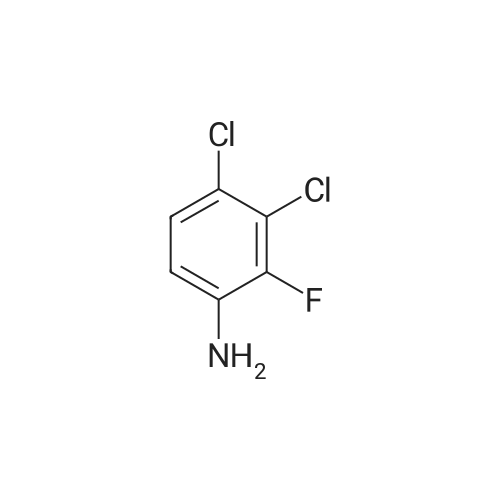
<strong>[179688-53-0]7-methoxy-4-oxo-3,4-dihydroquinazolin-6-yl acetate</strong> (100 g) was added to toluene (850 mL) and NN-diisopropylethylamine (82.5 mL). Phosphorus oxychloride (100 mL) was added thereto over 20 minutes at 75C, followed by stirring for 3 hours. Toluene (450 mL) and 3,4-dichloro-2-fluoroaniline (84.6 g) were added to the resulting mixture, followed by stirring for 2 hours. Upon completion of the reaction, the resulting mixture was cooled to 25C, and the solid thus obtained was filtered under a reduced pressure and washed with toluene (400 mL). Isopropanol (1,000 mL) was added to the solid, and the resulting mixture was stirred for 2 hours. The solid thus obtained was filtered and washed with isopropanol (400 mL), and then was dried at 40C in an oven to obtain the target compound (143 g, yield: 83%). 1H-NMR (DMSO-d6, 300 MHz, ppm) δ 8.92 (s, 1H), 8.76 (s, 1H), 7.69- 7.57 (m, 3H), 4.01 (s, 3H), 2.38 (s, 3H). |
| 83% |
|
Example 1 Preparation of 4-(3,4-dichloro-2-fluorophenylamino)-7-methoxyquinazolin-6-yl acetate (the compound of formula (VI)) 7-methoxy-4-oxo-3,4-dihydroquinazolin-yl acetate (100 g) was added to toluene (850 ml) and N,N-diisopropylethylamine (82.5 ml). Phosphorusoxy chloride (100 ml) was added thereto over 20 minutes at 75 C., followed by stirring for 3 hours. Toluene (450 ml) and 3,4-dichloro-2-fluoroaniline (84.6 g) were added to the resulting mixture, followed by stirring for 2 hours. Upon completion of the reaction, the resulting mixture was cooled to 25 C. The solid thus obtained was filtered under a reduced pressure and washed with toluene (400 ml). Isopropanol (1,000 ml) was added to the solid, which was then stirred for 2 hours. The resulting solid was filtered and washed with isopropanol (400 ml). The solid was dried at 40 C. in an oven to produce the compound of formula (VI) (143 g, yield: 83%). 1H-NMR (DMSO-d6, 300 MHz, ppm) δ 8.92 (s, 1H), 8.76 (s, 1H), 7.69-7.57 (m, 3H), 4.01 (s, 3H), 2.38 (s, 3H). |
| 83% |
|
100 g of <strong>[179688-53-0]7-methoxy-4-oxo-3,4-dihydroquinazolin-6-yl acetate</strong> was added to 850 ml of toluene and 82.5 ml of N, N-diisopropylethylamine,100 ml of phosphorus oxychloride was added at 75 DEG C for 20 minutes and then stirred for 3 hours.450 ml of toluene was further added to the resulting reaction solution, 84.6 g of 3,4-dichloro-2-fluoroaniline was added, and the mixture was stirred for 2 hours. When the reaction was completed, the temperature of the reaction solution was cooled to 25 C, and the resulting solid was filtered under reduced pressure and washed with 400 ml of toluene. 1000 ml of isopropanol was added to the obtained solid, and the mixture was stirred for 2 hours. The solid was filtered and washed with 400 ml of isopropanol.The obtained solid was dried in a drying oven at 40 to obtain the title compound (143 g, yield 83%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping