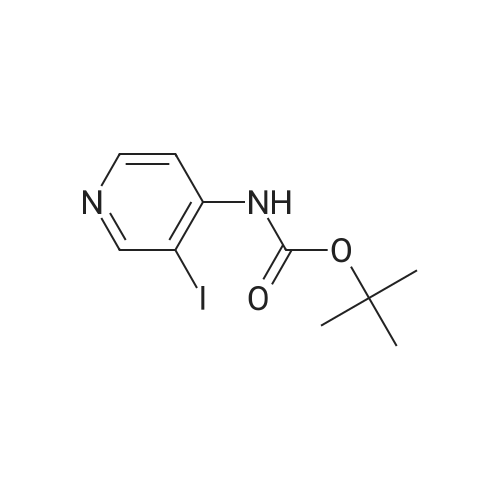
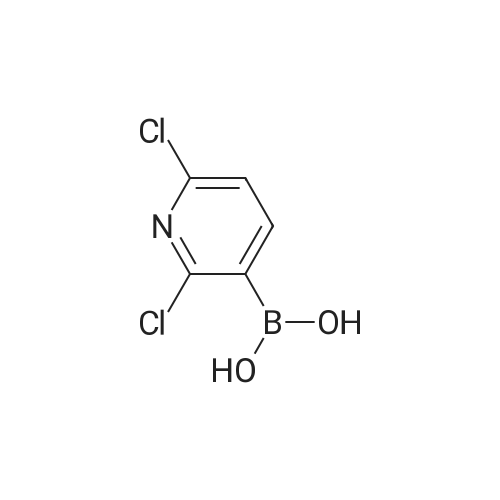
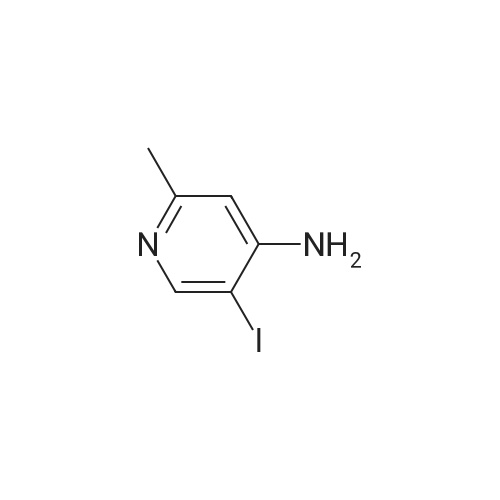
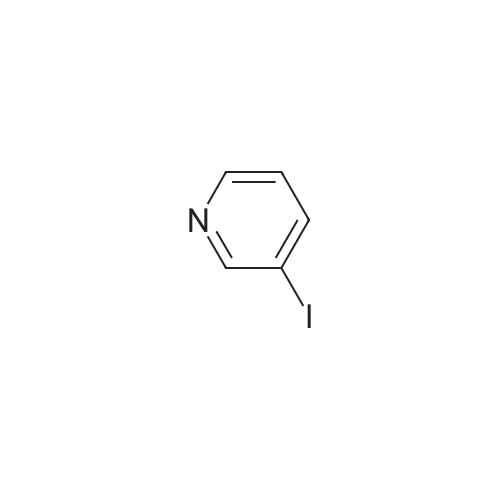
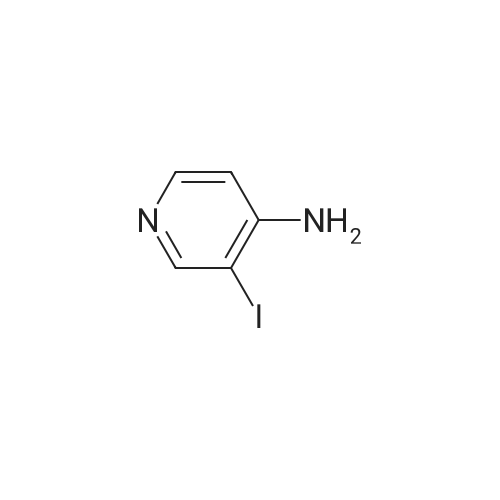
| 44% |
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In 1,4-dioxane; water; at 100℃; for 16h;Inert atmosphere; |
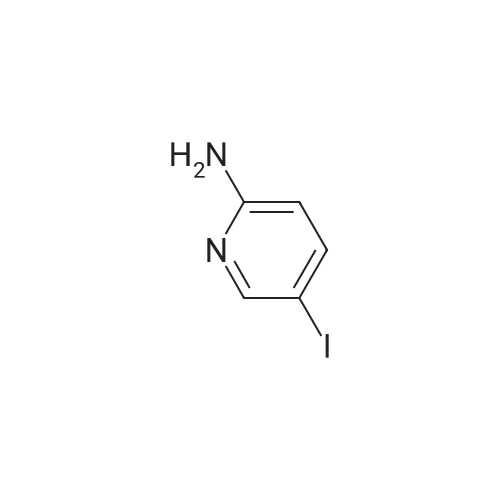
General procedure: Procedure A : Suzuki coupling To a solution of iodopyridine (1 eq) in dioxane (5 mL/mmol), the boronic acid (1.5 eq), and 1 M Na2C03 aqueous solution (3 eq) were added and the reaction mixture was degassed with argon for 20 min. Then Bis(triphenylphosphine)palladium(ll) dichloride (0.2 eq) was added and the reaction mixture was heated at 100C for 16h. After completion of reaction, the reaction mixture was filtered through a celite pad and the filtrate was concentrated under reduced pressure to afford a residue that was dissolved in water and extracted with ethyl acetate. The organic layer was separated, dried over sodium sulphate and concentrated under reduced pressure to afford the crude product, which was further purified by silica gel (100:200 mesh) column chromatography to afford the Suzuki coupling product. |
| 44% |
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In 1,4-dioxane; water; at 100℃; for 16h;Inert atmosphere; |
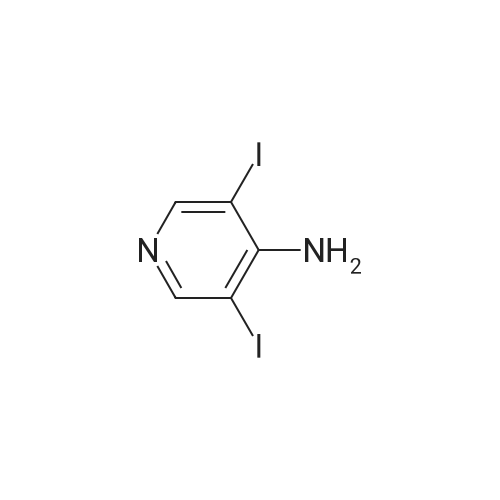
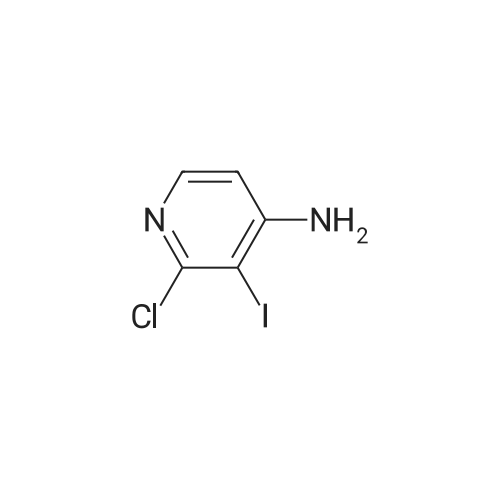
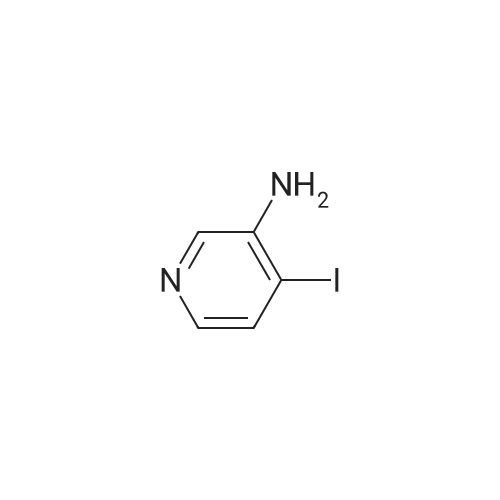
To a solution of 3-iodopyridin-4-amine (6 g, 27.2 mmol) in dioxane (135 ml_), (2,6-dichloropyridin- 3-yl)boronic acid (7.29 g, 38.1 mmol), and 1 M Na2CC>3 aqueous solution (3 eq) were added and the reaction mixture was degassed with argon for 20 min. Then Bis(triphenylphosphine)palladium(ll) dichloride (3.79 g, 5.4 mmol) was added and the reaction mixture was heated at 100C for 16h. After completion of reaction, the reaction mixture was filtered through a celite pad and the filtrate was concentrated under reduced pressure to afford a residue that was dissolved in water and extracted with ethyl acetate. The organic layer was separated, dried over sodium sulphate and concentrated under reduced pressure to afford the crude product, which was further purified by silica gel (100:200 mesh) column chromatography to afford 2',6'-dichloro-[3,3'-bipyridin]-4-amine (i1 ) (2.9 g, Yield 44%). (0139) 1H NMR (400 MHz, DMSO-de) delta 6.04 (s, 2H), 6.62 (d, J = 5.8 Hz, 1 H), 7.71 - 7.55 (m, 1 H), 7.94 - 7.75 (m, 2H), 8.03 (d, J = 5.7 Hz, 1 H). (0140) MS (ESI) m/e (M+1 )+: 240.05 |
| 44% |
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In 1,4-dioxane; water; at 100℃; for 16h;Inert atmosphere; |
To a solution of 3-iodopyridin-4-amine (6 g, 27.2 mmol) in dioxane (135 mL), <strong>[148493-34-9](2,6-dichloropyridin-3-yl)boronic acid</strong> (7.29 g, 38.1 mmol), and 1M Na2CO3 aqueous solution (3 eq) were added andthe reaction mixture was degassed with argon for 20 mm. ThenBis(triphenylphosphine)palladium(ll) dichloride (3.79 g, 5.4 mmol) was added and the reactionmixture was heated at 100C for 16h. After completion of reaction, the reaction mixture wasfiltered through a celite pad and the filtrate was concentrated under reduced pressure to afford a residue that was dissolved in water and extracted with ethyl acetate. The organic layer was separated, dried over sodium sulphate and concentrated under reduced pressure to afford the crude product, which was further purified by silica gel (100:200 mesh) column chromatography toafford 2,6-dichloro-[3,3-bipyridin]-4-amine (ii) (2.9 g, Yield 44%).1H NMR (400 MHz, DMSO-d6) 66.04 (s, 2H), 6.62 (d, J= 5.8 Hz, 1H), 7.71 -7.55 (m, 1H), 7.94- 7.75 (m, 2H), 8.03 (d, J = 5.7 Hz, 1 H).MS (ESI) m/e (M+1): 240.05 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping