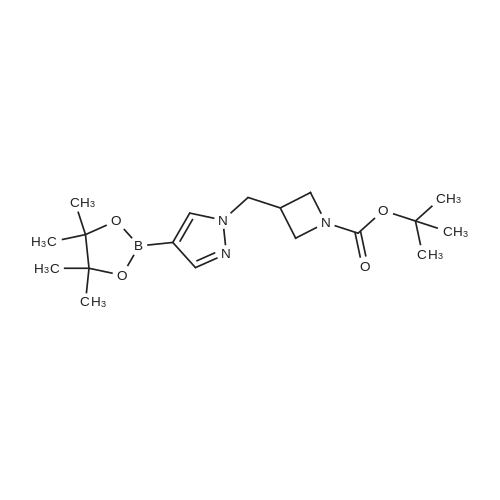
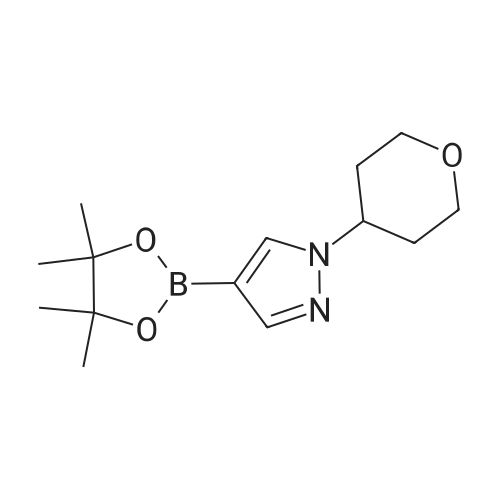
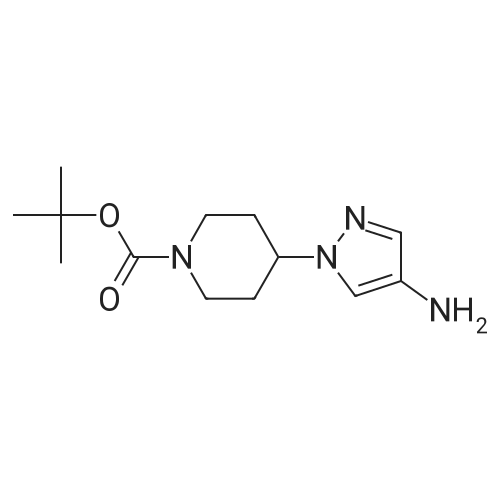
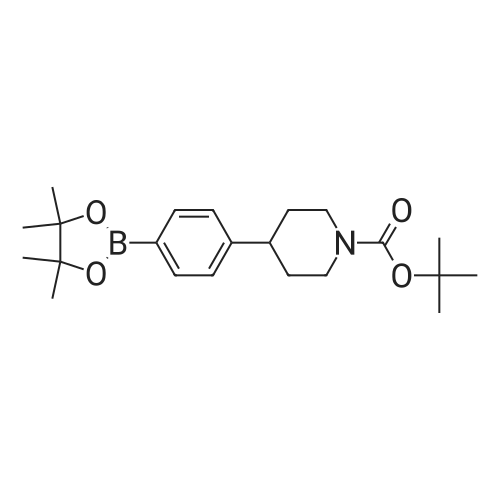
| 81% |
With isopropylmagnesium chloride; In tetrahydrofuran; at -10 - 30℃; for 9h;Inert atmosphere; Large scale; |
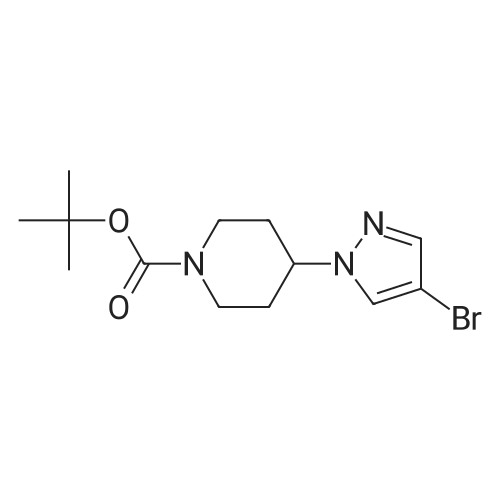
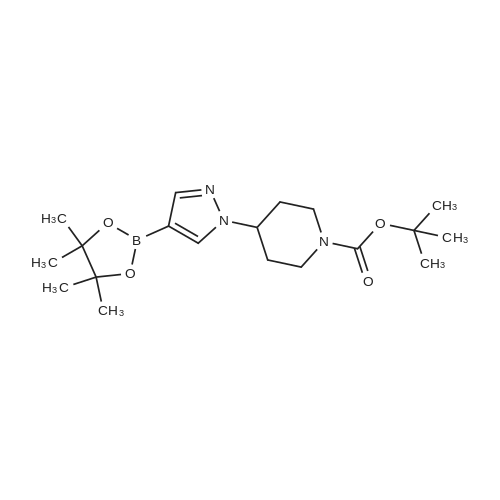
1.3 kg of compound (CZT-8) was dissolved in 6.5 liters of dry tetrahydrofuran,Nitrogen protection,Down to -10 degrees,A solution of 3.2 liters of 2N isopropylmagnesium chloride in tetrahydrofuran was slowly added,After the completion of heating to 20 degrees,Add 1.0 publicjinEven boronic acid6.5 liters of tetrahydrofuran solution,Control the temperature between 20 degrees to 30 degrees,After the completion of the reaction at room temperature for 9 hours.Add 9 liters of ethyl acetate and 10 liters of water,Stir for 2 hours,Dispensing,Dried and concentrated.Recrystallization gave 1.2 kg of a white solid(CZT-9). Yield 81% |
| 44% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In dimethyl sulfoxide; at 80℃; for 0.166667h;Inert atmosphere; |
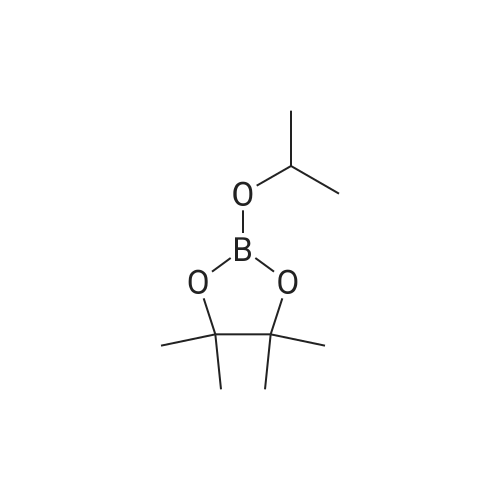
C. A mixture of tert-butyl 4-(4-bromo- lH-pyrazol- 1 -yl)piperidine- 1-carboxylate (20.0 g, 0.61 mmol), 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)-l,3,2-dioxaborolane (30.8 g, 0.12 mmol) and potassium acetate (17.8 g, 0.18 mol) in 50 mL of dimethyl sulfoxide was purged with nitrogen gas for 10 min. After the addition of [l, -bis(diphenylphosphino)ferrocene]dichloropalladium(II) (3.55 g, 4.85 mmol), the mixture was purged with nitrogen gas for another 10 minutes, heated at 80 C overnight under nitrogen atmosphere and filtered through celite and washed with ethyl acetate. The filtrate was extracted with ethyl acetate (2 x 200 mL). The organic layer was dried over anhydrous sodium sulfate. After filtration and removal of the solvent, the residue was purified by column chromatograph eluted with hexane to afford an oil which was recrystallized from hexane to afford tert-butyl 4-(4-(4,4,5,5- tetramethyl- 1 ,3 ,2-dioxaborolan-2-yl)- lH-pyrazol- 1 -yl)piperidine- 1 -carboxylate as a white solid in 44% yield (10 g). 1H NMR (400 MHz, CDC13) delta 7.79 (s, 1H), 7.72 (s, 1H), 4.32-4.13 (m, 3H), 2.95-2.80 (m, 2H), 2.15-2.05 (m, 2H), 1.93-1.82 (m, 2H), 1.47 (s, 9H), 1.31 (s, 12H). |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 90℃;Inert atmosphere; |
As shown in step 3-iii of Scheme 3, tert-butyl 4-(4-bromo-lH-pyrazol-l- yl)piperidine-l-carboxylate (Compound 1014, 10.52 g, 31.86 mmol), 4,4,5, 5-tetramethyl-2- (4,4,5, 5-tetramethyl-l,3,2-dioxaborolan-2-yl)-l,3,2-dioxaborolane (9.71 g, 38.23 mmol), and potassium acetate (9.38 g, 95.58 mmol) were taken up in 105 mL of 1,4-dioxane. The mixture was flushed with nitrogen for 20 minutes and PdCl2(dppf) (1.3 g, 1.59 mmol) was added. The reaction was heated at 900C for 11 hours. The reaction was cooled to room temperature and filtered through a plug of Florisil, which was subsequently rinsed with ethyl acetate. The filtrate was concentrated under reduced pressure to afford a dark brown oil that was dissolved in hexanes and eluted through a second plug of Florisil with 1 :2 EtOAc/hexanes. The filtrate was concentrated under reduced pressure to give a tan oil, which was triturated with hexanes and stirred at 00C until a white precipitate formed. The precipitate was collected by vacuum filtration, washed with hexanes, and dried to afford 6.79 g of tert-butyl 4-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazol-l-yl)piperidine- 1-carboxylate (Compound 1015). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping