| 81% |
With potassium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In tetrahydrofuran; water; at 35℃; for 24h;Inert atmosphere; |
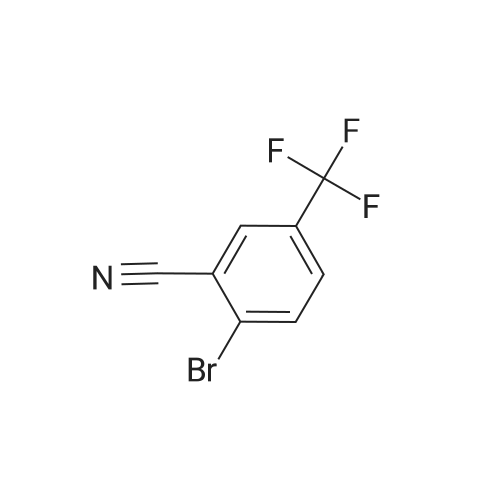
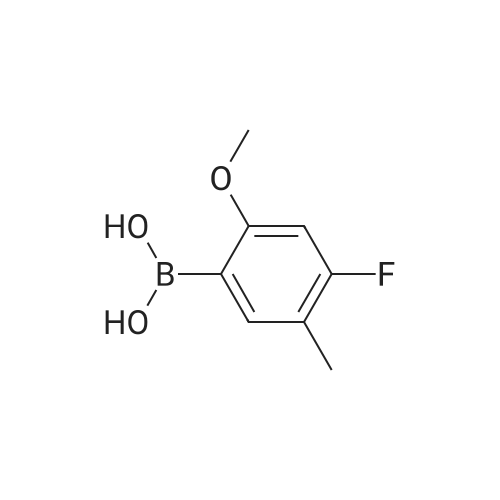
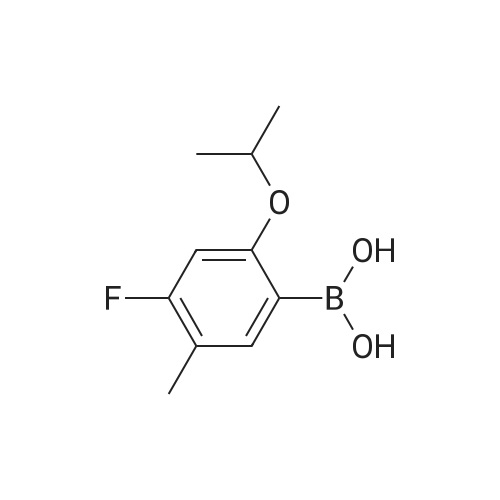
K2CO3 (3.32 g, 24 mmol) is dissolved in water (20 mL) and the resulting solution is degassed by sparging with argon gas for 10 min. (2-chloro-5-(trifluoromethyl)phenyl) methanol (COK) (2.94 g, 14 mmol), and boranic acid METB (2.78 g, 14 mmol) dissolved in THF (20 mL) are added to the K2CO3 solution. The resulting solution is degassed by sparging with argon gas for 15 min. The catalyst, 1,1 bis(di-tertbutylphosphino)ferrocene palladium dichloride (75 mg, 0.8 mol%) is added. The organic layer turns dark brown immediately. The biphasic mixture is aged at 35 C with vigorous stirring for 24 hours. The mixture is cooled to rt and water (80) is added, followed by DIPE (80 mL) and the aqueous layer is removed. The organic layer was washed with 1 M NaOH (aq) (50 mL), 1 M HCl (aq) (50 mL) and water (50 mL), dried over Na2SO4, and filtered through silica gel pot The solvent is removed under reduced pressure to yield EBFOH as a brownish solid (4.18 g, 91%):'H NMR (CDCl3) delta 1.22 (t, J = 7.6, 3H), 1.95 (t, J= 6.2,1H), 2.64 (q, J =7.5, 2H), 4.49 (bs, 2H), 6.69 (d, J =11.6, 1H), 6.96 (d, J = 8.7, 1H), 7.29 (d, J = 7.9, 1H), 7.58 (d, J = 7.9, 1H), 7.85 (s, 1H). |
| 81% |
With potassium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In tetrahydrofuran; water; at 20 - 45℃; for 32h;Inert atmosphere;Product distribution / selectivity; |
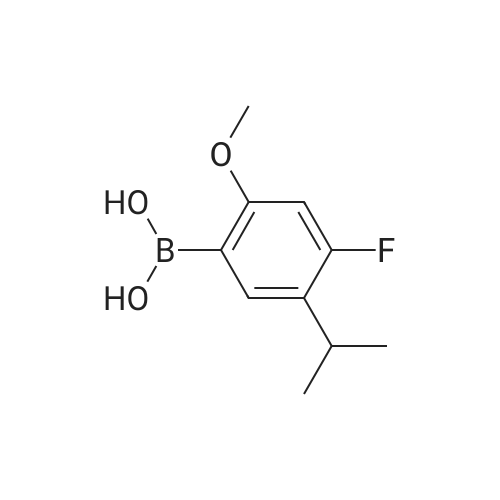
A 3 M K2CO3 solution is prepared by adding solid K2CO3 (31 g, 0.22 mol) to water (100 mL). Cooling is applied to keep the solution at 20-25 C. (2-chloro-5-(trifluoromethyl)phenyl)methanol (Formula VI', X = Cl, R1 = CH2OH) (17.5 g, 84 mmol), and 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid (MIPB) (18.1 g, 85 mmol) are added to the K2C03 followed by all THF (100 mL) rinse. The solution is degassed by sparging with argon gas for 20 min. The catalyst, 1,1-bis(di-tertbutylphosphino)ferrocene palladium dichloride (300 mg, 0.55 mol%) is added. The organic layer turns dark brown immediately. The biphasic mixture is aged at 35-45 C with vigorous stirring for 32 hours. The mixture is cooled to r. t. and water (150 mL) is added, followed by petrol ether (150 mL) and the aqueous layer is removed. The organic layer was washed with water (2×200 mL) and filtered through silica gel and the solvent is removed under reduced pressure to yield brownish oil which is crystallized from heptane to give 4'-fluoro-5'-isopropyl-2'-methoxy-4-(trifluoromethyl)biphenyl-2-yl)methanol as a pale white solid (28.5 g, 80%). mp 93,5-95,5 C; 1H NMR (CDCl3) delta 1.24 (d, J = 6.9 Hz, 6H), 1.95 (t, J = 6.1 Hz, 1H), 3.21 (sept., J = 6.9 Hz, 1H), 3.73 (s, 3H), 4.49 (m, 2H), 6.68 (d, J = 12.0 Hz, 1H), 6.99 (d, J = 8.6 Hz, 1H), 7.30 (d, J = 7.9 Hz, 1H), 7.59 (dd, J1 = 8.0 Hz, J2 =1.3 Hz, 1H), 7.86 (d, J = 0.7 Hz, 1H). According to the same procedure in various scales and different concentrations of catalyst some other biaryl of Formula VII1 and VII2 are prepared and listed in Table 1 and Table 2, respectively. |
| 81% |
With dichloro[1,1'-bis(di-t-butylphosphino)ferrocene]palladium(II); potassium carbonate; In tetrahydrofuran; water; at 20 - 45℃; for 32h;Inert atmosphere; |
General procedure: A 3 M K2CO3 solution is prepared by adding solid K2C03 (31 g, 0.22 mol) to water (100 mL). Cooling is applied to keep the solution at 20-25 C. (2-chloro-5-(trifluoromethyl)phenyl)methanol (Formula VI', X = CI, Ri = CH2OH) (17.5 g, 84 mmol), and 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid (MIPB) (18.1 g, 85 mmol) are added to the K2CO3 followed by all THF (100 mL) rinse. The solution is degassed by sparging with argon gas for 20 min. The catalyst, 1 , 1-bis(di-tertbutylphosphino)ferrocene palladium dichloride (300 mg, 0.55 mol%) is added. The organic layer turns dark brown immediately. The biphasic mixture is aged at 35-45 C with vigorous stirring for 32 hours. The mixture is cooled to r. t. and water (150 mL) is added, followed by petrol ether (150 mL) and the aqueous layer is removed. The organic layer was washed with water (2x200 mL) and filtered through silica gel and the solvent is removed under reduced pressure to yield brownish oil which is crystallized from heptane to give 4'-fluoro-5'-isopropyl-2'-methoxy-4-(tnfluoromethyl)biphenyl-2-yl)methanol as a pale white solid (28.5 g, 80%). mp 93.5-95.5 C; 1H NMR (CDC ) C 1 .24 (d, J = 6.9 Hz, 6H), 1.95 (t, J = 6.1 Hz, 1 H), 3.21 (sept., J = 6.9 Hz, 1 H), 3.73 (S, 3H), 4.49 (m, 2H), 6.68 (d, J = 12.0 Hz, 1 H), 6 99 (d, J = 8.6 Hz, 1 H), 7.30 (d, J = 7.9 Hz, 1 H), 7.59 (dd, J, = 8.0 Hz, J2 = 1 .3 Hz, 1 H), 7.86 (d, J = 0.7 Hz, 1 H). According to the same procedure in various scales and different concentrations of catalyst some other biaryl of Formula VII1 and Vll2 are prepared and listed in Table 1 and Table 2, respectively. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping