| 52% |
|
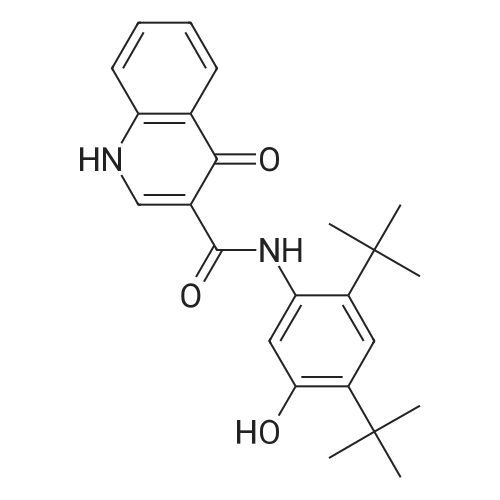
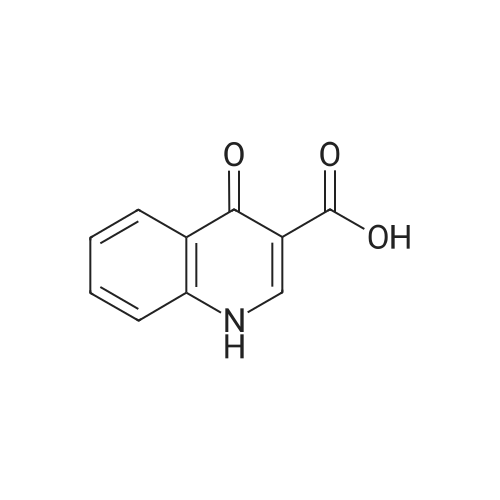
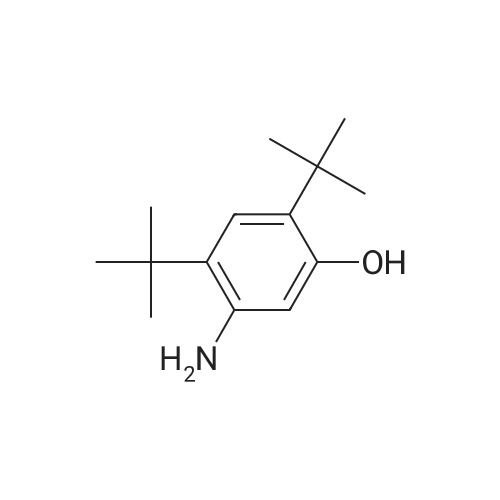
Step E: N-(5-hydroxy-2,4-di-tert-butyl-phenyl)-4-oxo-1H-quinoline-3-carboxamide To a suspension of 4-oxo-1,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (2.80 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5-amino-2,4-di-tert-butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+ 190, 1.71 min), the solvent was removed in vacuo. EtOH was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C.) for 15 min without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystalate. Et2O was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovapor (bath temperature 25 C.) for 15 min without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide 1-(5-hydroxy-2,4-di-tert-butyl-phenyl)-4-oxo-1H-quinoline-3-carboxamide as a white powdery solid (38 g, 52%). HPLC ret. time 3.45 min, 10-99% CH3CN, 5 min run; 1H NMR (400 MHz, DMSO-d6) delta 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J=8.2, 1.0 Hz, 1H), 7.83-7.79 (m. 1H), 7.76 (d, J=7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H) ppm; ESI-MS m/z calc'd 392.21; toured 393.3 [M+H]+. |
| 52% |
|
1003431 To a suspension of 4-oxo-1,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 mm before 5-amino-2,4-di-tert-butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+ 190, 1.71 mm), the solvent was removed in vacuo. EtOH was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C) for 15 mm without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystalate. Et20 was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovapor (bath temperature 25 C) for 15 mm without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide N-(5-hydroxy-2,4-di- tert-butyl-phenyl)-4-oxo-1H-quinoline-3-carboxamide as a white powdery solid (38 g, 52%). HPLC ret. time 3.45 mm, 10-99% CH3CN, 5 mm run; ?H NIVIR (400 IVIHz, DMSO-d6) 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J 8.2, 1.0 Hz, 1H), 7.83-7.79 (m, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H); ESI-MS m/z calc?d 392.21; found 393.3 [M+H]t |
| 52% |
|
To a suspension of 4-oxo-l,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5-amino-2,4-di-ie/ -butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+ 190, 1.71 min), the solvent was removed in vacuo. EtOH was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C) for 15 min without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystalate. Et20 was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovap (bath temperature 25 C) for 15 min without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide N-(5-hydroxy-2,4-di- ieri-butyl-phenyl)-4-oxo-lH-quinoline-3-carboxamide as a white powdery solid (38 g, 52%). HPLC ret. time 3.45 min, 10-99% CH3CN, 5 min run; lH NMR (400 MHz, DMSO-ifc) delta 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J = 8.2, 1.0 Hz, 1H), 7.83-7.79 (m, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H); ESI-MS m z calc'd 392.21; found 393.3 [M+H]+. |
| 52% |
|
To a suspension of 4-oxo-l,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5-amino-2,4-di-ie/t-butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+ 190, 1.71 min), the solvent was removed in vacuo. EtOH was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C) for 15 min without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystallate. Et20 was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovap (bath temperature 25 C) for 15 min without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide N-(5-hydroxy-2,4-di-ieri-butyl-phenyl)-4-oxo-lH-quinoline-3- carboxamide as a white powdery solid (38 g, 52%). HPLC ret. time 3.45 min, 10-99% CH3CN, 5 min run; lH NMR (400 MHz, DMSO-d6) delta 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J = 8.2, 1.0 Hz, 1H), 7.83-7.79 (m, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H); ESI- MS m/z calc'd 392.21 ; found 393.3 [M+H]+. |
| 52% |
|
To a suspension of 4-oxo-l,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5-amino-2,4-di-ie/ -butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+ 190, 1.71 min), the solvent was removed in vacuo. EtOH was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C) for 15 min without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystalate. Et20 was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovapor (bath temperature 25 C) for 15 min without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide N-(5-hydroxy-2,4-di- ieri-butyl-phenyl)-4-oxo-lH-quinoline-3-carboxamide as a white powdery solid (38 g, 52%). HPLC ret. time 3.45 min, 10-99% CH3CN, 5 min run; lH NMR (400 MHz, DMSO-ifc) delta 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J = 8.2, 1.0 Hz, 1H), 7.83-7.79 (m, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H); ESI-MS m z calc'd 392.21; found 393.3 [M+H]+. |
| 52% |
|
To a suspension of 4-oxo-l,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5-amino-2,4-di-/
|
| 52% |
|
To a suspension of 4-oxo-1,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5-amino-2,4-di-tert-butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+190, 1.71 min), the solvent was removed in vacuo. EtOH (ethyl alcohol) was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C.) for 15 min without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystalate. Et2O (diethyl ether) was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovapor (bath temperature 25 C.) for 15 min without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide N-(5-hydroxy-2,4-di-tert-butyl-phenyl)-4-oxo-1H-quinoline-3-carboxamide (38 g, 52%). HPLC ret. time 3.45 min, 10-99% CH3CN, 5 min run; 1H NMR (400 MHz, DMSO-d6) delta 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J=8.2, 1.0 Hz, 1H), 7.83-7.79 (m, 1H), 7.76 (d, J=7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H); ESI-MS m/z calc'd 392.21; found 393.3 [M+H]+. |
| 52% |
|
To a suspension of 4-oxo-1,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5 amino-2,4-di-tert-butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+ 190, 1.71 min), the solvent was removed in vacuo. EtOH (ethyl alcohol) was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C) for 15 min without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystalate. Et20 (diethyl ether) was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovapor (bath temperature 25 C) for 15 min without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide N-(5-hydroxy-2,4-di-ie - butyl-phenyl)-4-oxo-lH-quinoline-3-carboxamide (38 g, 52%). HPLC ret. time 3.45 min, 10-99% CH3CN, 5 min run; 1H NMR (400 MHz, DMSO-d6) d 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J = 8.2, 1.0 Hz, 1H), 7.83-7.79 (m, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H); ESI-MS m/z calc'd 392.21; found 393.3 [M+H]+. |
| 52% |
|
To a suspension of 4-oxo-1,4-dihydroquinolin-3-carboxylic acid (35.5 g, 188 mmol) and HBTU (85.7 g, 226 mmol) in DMF (280 mL) was added Et3N (63.0 mL, 451 mmol) at ambient temperature. The mixture became homogeneous and was allowed to stir for 10 min before 5-amino-2,4-di-tert-butyl-phenol (50.0 g, 226 mmol) was added in small portions. The mixture was allowed to stir overnight at ambient temperature. The mixture became heterogeneous over the course of the reaction. After all of the acid was consumed (LC-MS analysis, MH+190, 1.71 min), the solvent was removed in vacuo. EtOH (ethyl alcohol) was added to the orange solid material to produce a slurry. The mixture was stirred on a rotovap (bath temperature 65 C.) for 15 min without placing the system under vacuum. The mixture was filtered and the captured solid was washed with hexanes to provide a white solid that was the EtOH crystalate. Et2O (diethyl ether) was added to the solid obtained above until a slurry was formed. The mixture was stirred on a rotovapor (bath temperature 25 C.) for 15 min without placing the system under vacuum. The mixture was filtered and the solid captured. This procedure was performed a total of five times. The solid obtained after the fifth precipitation was placed under vacuum overnight to provide N-(5-hydroxy-2,4-di-tert-butyl-phenyl)-4-oxo-1H-quinoline-3-carboxamide (38 g, 52%). HPLC ret. time 3.45 min, 10-99% CH3CN, 5 min run; 1H NMR (400 MHz, DMSO-d6) delta 12.88 (s, 1H), 11.83 (s, 1H), 9.20 (s, 1H), 8.87 (s, 1H), 8.33 (dd, J=8.2, 1.0 Hz, 1H), 7.83-7.79 (m, 1H), 7.76 (d, J=7.7 Hz, 1H), 7.54-7.50 (m, 1H), 7.17 (s, 1H), 7.10 (s, 1H), 1.38 (s, 9H), 1.37 (s, 9H); ESI-MS m/z calc'd 392.21; found 393.3 [M+H]+. |
| 46% |
|
Examples: 3: Syn (0087) (0088) A mixture of 4-oxo-l,4-dihydroquinoline-3-carboxylic acid 3 (0.25 g, 1.32 mmol), N,N- diisopropylethylamine (0.97 mL, 5.38 mmol), and HATU (1.01 g, 2.64 mmol) in DMF (5 mL) was stirred at 25 C for 10 min and then 5-amino-2,4-di-tert-butylphenol 3a (0.58 g, 2.64 mmol) was added in one portion, allowed to stir forl2h. The reaction mass was extracted with EtOAc (2 X 10 mL). Combined organic layers were washed with H20 (5 mL), saturated NaHC03 solution (5 mL), H20 (5 mL), brine (5 mL), dried over anhydrous Na2S04 and evaporated to dryness, crude residue was purified by silica gel chromatography (2-3% MeOH-DCM) to give off-white solid, which on further crystallization in EtOH gave desired compound as white solid (0.23 g, 46%). (0089) 1H NMR (500MHz ,DMSO-d6) delta = 12.88 (brs, 1H), 11.82 (brs, 1H), 9.20 (brs, 1H), 8.87 (brs, 1H), 8.33 (d, J = 6.9 Hz, 1H), 7.88 - 7.68 (m, 2H), 7.52 (brs, 1H), 7.17 (brs, 1H), 7.11 (brs, 1H), 1.38 (d, J = 7.6 Hz, 18H). |
|
|
A mixture of 4-oxo-l,4-dihydroquinoline-3-carboxylic acid (lO.Ogm), HATU (24.12 gm), Di- isopropyl ethyl amine (20.56 ml) and DMF (60.0ml) was stirred for 30 minutes. 5-amino-2-4-di- tert-butyl-phenol (12.8 gm) was added to the reaction mass and stirred for a period of about 15.0 hr. Water was added to the reaction mass and product was extracted with ethyl acetate. Organic layer was washed with 10% sodium carbonate solution followed by brine. Organic layer was distilled under vacuum. 100ml of methanol was added to obtained residue and heated to 60-65C for 30min. The solid was filtered and dried at 50-55C for 12 hrs to obtain 15.1gm of title compound, (DSC) thermogram having endothermic peak at about 320.88, 192 .13±1C and exothermic peak at245.63±lC water content (by K.F): 0.34% , HPLC purity: 99.86% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping