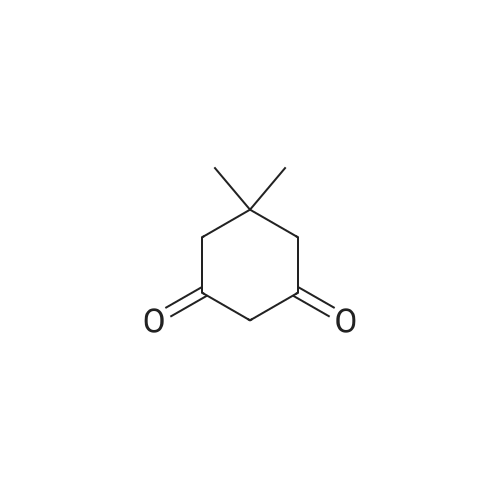
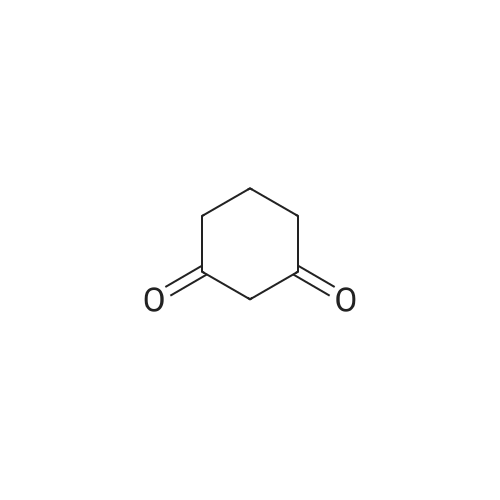
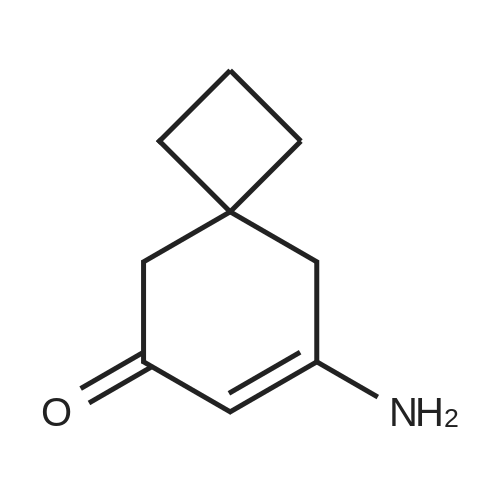
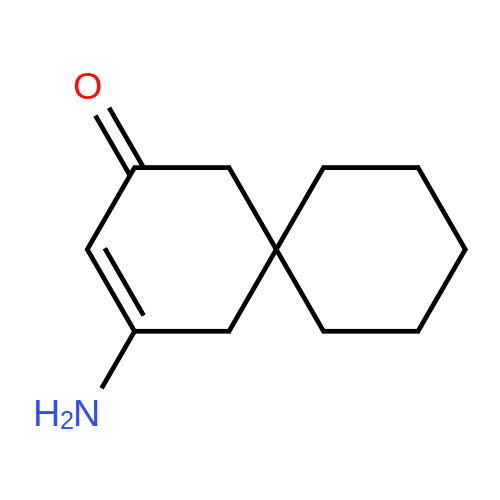
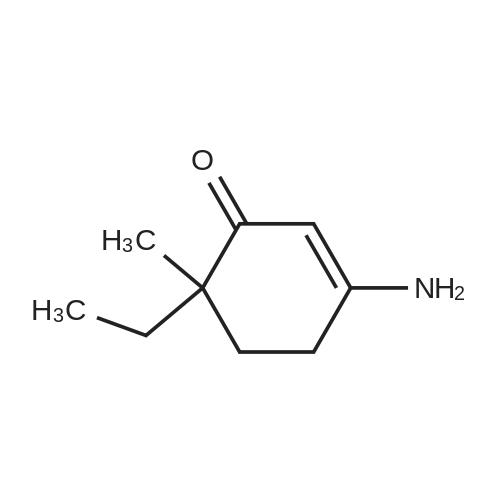
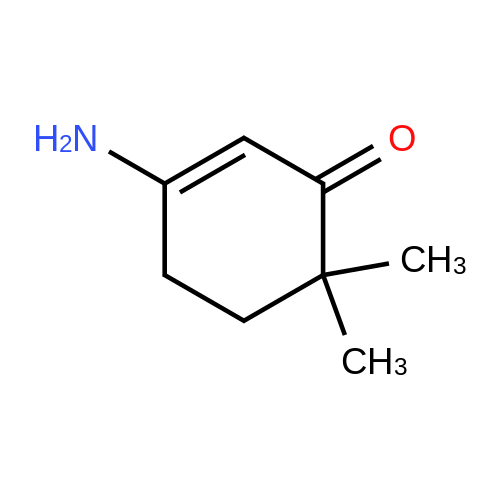
| 86% |
With ammonium acetate; acetic acid; In toluene; for 5h;Reflux; Dean-Stark; |
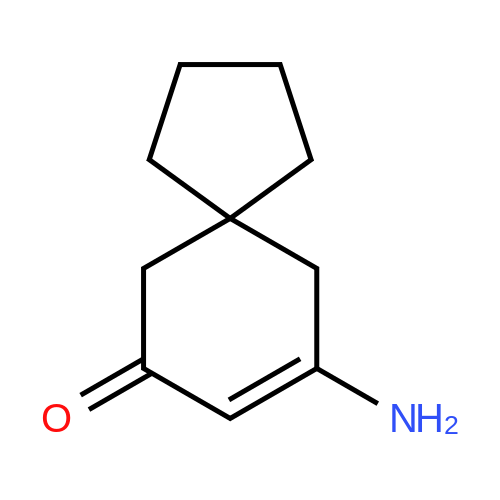
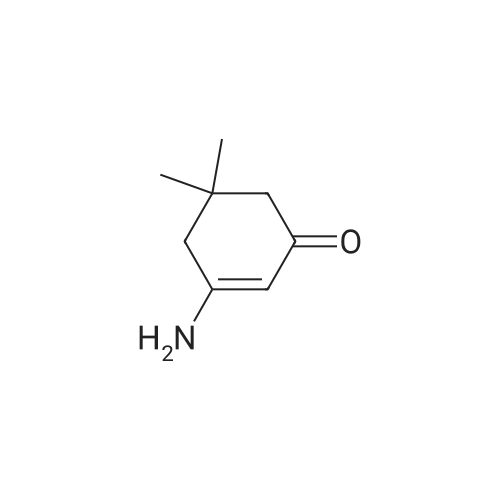
3-Amino-5,5-dimethyl-2-cyclohexen-1 -one 3-Amino-5,5-dimethyl-2-cyclohexen-1 -one can be synthesized as known from literature or can be obtained by the following synthesis: A mixture of 200 g dimedone, 143 g ammonium acetate, and 5 ml acetic acid in 2 L toluene is refluxed for 5 h at a Dean-Stark trap. Then the solution is cooled to room temperature and stirred for 16 h. The suspension is filtered and the filter cake is dried at 65C. Yield: 171 g (86 % of theory) Mass spectrometry (ESI+): m/z = 140 [M+H]+ Rf-value: 0.31 (silica gel, dichloromethane/ethanol 9:1 ) |
| 86% |
With ammonium acetate; In acetic acid; toluene; at 20℃; for 21h;Reflux; Dean-Stark; |
3-Amino-5,5-dimethyl-2-cyclohexen-1-one can be synthesized as known from literature or can be obtained by the following synthesis: [0223] A mixture of 200 g dimedone, 143 g ammonium acetate, and 5 ml acetic acid in 2 L toluene is refluxed for 5 h at a Dean-Stark trap. Then the solution is cooled to room temperature and stirred for 16 h. The suspension is filtered and the filter cake is dried at 65 C. [0224] Yield: 171 g (86% of theory) [0225] Mass spectrometry (ESI+): m/z=140 [M+H]+ [0226] Rf-value: 0.31 (silica gel, dichloromethane/ethanol 9:1) |
| 85% |
With ammonium acetate; In toluene; for 5h;Reflux; |
5,5-dimethylcyclohexane-1,3-dione 10 (7.0 g,50 mmol) was added to a mixture of ammonium acetate (3.85 g, 50 mmol) in dry toluene(100 mL). The mixture was heated for 5 h under reflux using a Dean-Stark water separator.The red oily layer formed was then separated and recrystallized with ethyl acetate to afford3-amino-5,5-dimethylcyclohex-2-enone 11 (5.9 g, yield 85%) as a yellow solid. 1H-NMR (300 MHz,DMSO-d6)δ 6.67 (s, 2H), 4.90 (s, 1H), 2.11 (s, 2H), 1.90 (s, 2H), 0.96 (s, 6H). |
|
With ammonium acetate; acetic acid; In benzene; for 5h;Reflux; |
The title compound was prepared in a similar manner as described by P.G. Baraldi, Synthetic Communications, 1983, 902. To a suspension of 5,5-dimethylcyclohexane-1,3-dione (10 g, 71 .3 mmol) in benzene (180 mL) were added acetic acid (1.84 mL, 31.2 mmol) and ammonium acetate (11.0 g, 143 mmol). The reaction mixture was refluxed for 5 h and the water formed was removed azeotropically using a Dean-Stark apparatus. Subsequently the reaction mixture was cooled to RT, quenched with sat. aq. Na2CO3 and iN aq. NaOH and extracted with CH2CI2 several times. The combined organic extracts were dried (Na2504), filtered and concentrated to afford the title compound. MS (UPLCMS): 139.9 [M+H]+; tR (HPLC conditions i): 0.48 mm |
|
With ammonium acetate; In toluene; for 3h;Reflux; Dean-Stark; |
General procedure: 10 mmol cyclic 1,3-diketone wasadded to a mixture of 10 mmol ammonium acetate in dry toluene (20 mL). Themixture was heated for 3 h under reux using a Dean-Stark water separator. Theresulting oily product was separated and recrystallized with ethylacetate to give the corresponding enaminone as crystals. The enaminone productwas dissolved in 50ml THF and 2 equiv. of pyridine were added. To the mixedsolution, acetyl chloride (2 equiv.) in 5ml THF was added dropwise at 0 C. Thereaction mixture was warmed up to ambient temperature and stirred additional 5h at 60oC. The solvent was removed undervacuum and the crude product purified by flash chromatography |
|
With ammonium acetate; In neat (no solvent); at 90℃; for 0.5h;Green chemistry; |
General procedure: The SBA-IL (0.02 g) was activated in vacuum at 100 C and then after cooling to room temperature, dimedone 7 (1.5 mmol) and ammonium acetate 8 (2.4 mmol) were added to it. In solvent-free conditions, the whole reaction mixture was heated at 90 C for 30 min. After completion of the first step and without any work-up, Meldrum’s acid 10 (1.5 mmol) and aldehyde 11a-i (1.5 mmol) were added to the reaction mixture, as reported in Table 2, at 90 C. The completion of two steps of the reaction was indicated by TLC, the resulting solid product was dissolved in hot ethanol, filtered to remove the unsolvable catalyst, and the filtrate was cooled to afford the pure product. The spectroscopic and analytical data for new compounds are presented in the following section. |
|
|
General procedure: A mixture of dimedone or cyclohexane-1,3-dione (1 mmol), ammonium acetate(1.5 mmol) was heated at 100 C for appropriate time. After completion of the reactionindicated by TLC (1-2 h), the reaction mixture was cooled to room temperature.By addition of a few drops of chloroform, the yellow precipitate was obtained, whichwas purified by recrystallization from n-hexane/ethyl acetate, m. p. = 157-159 Cand m. p. = 132-134 C, respectively |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping