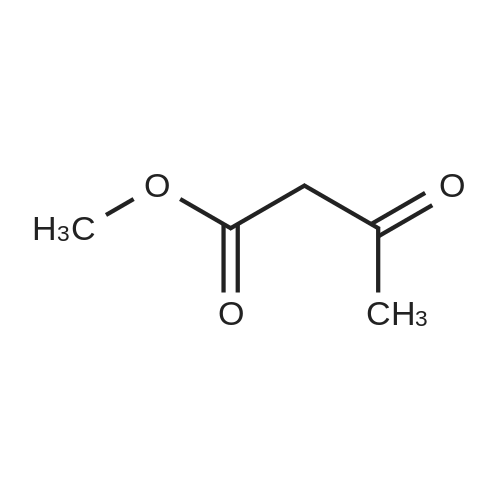
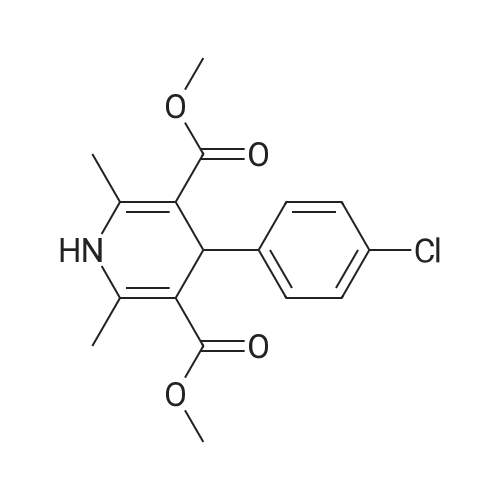
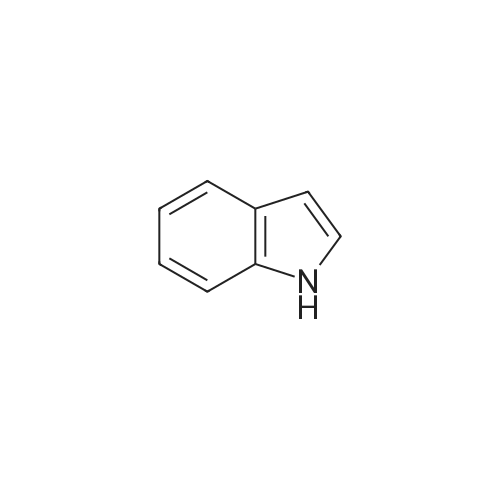
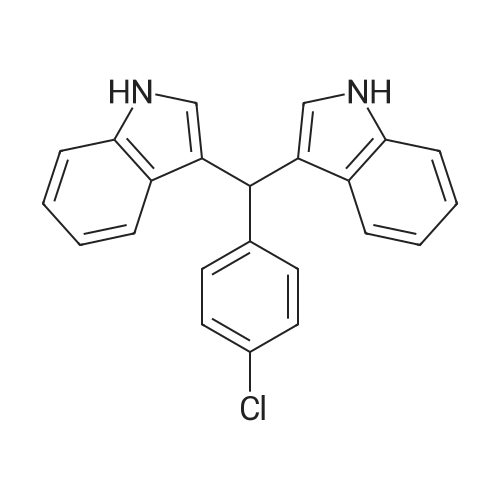
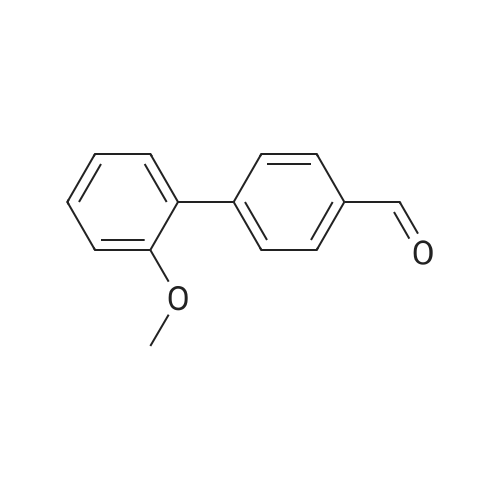
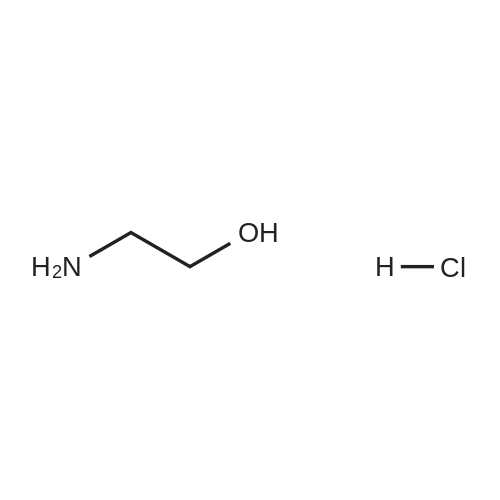
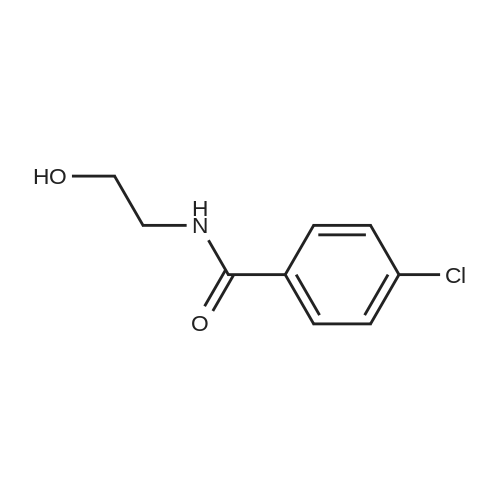
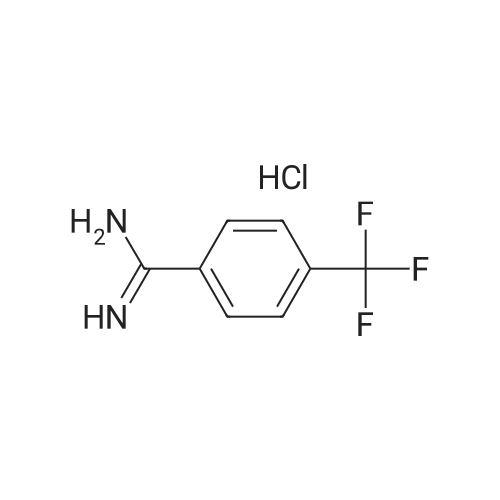
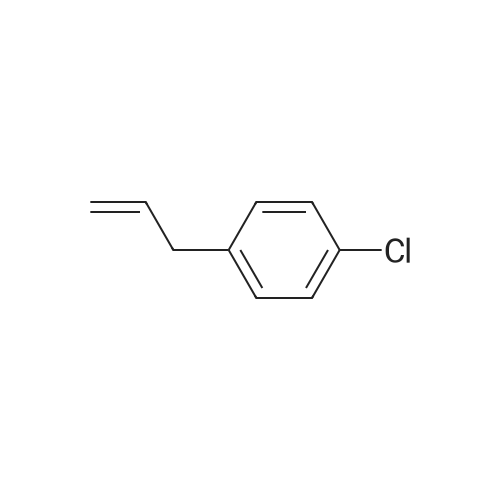
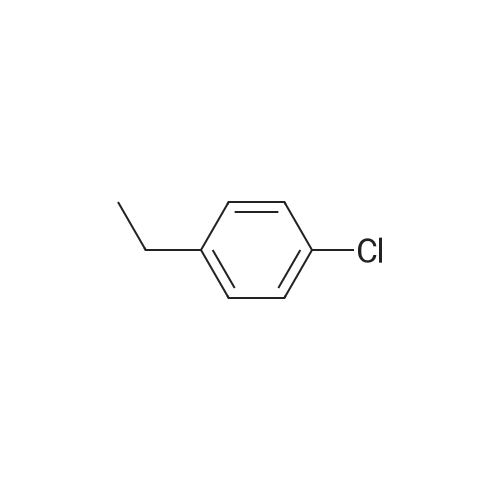
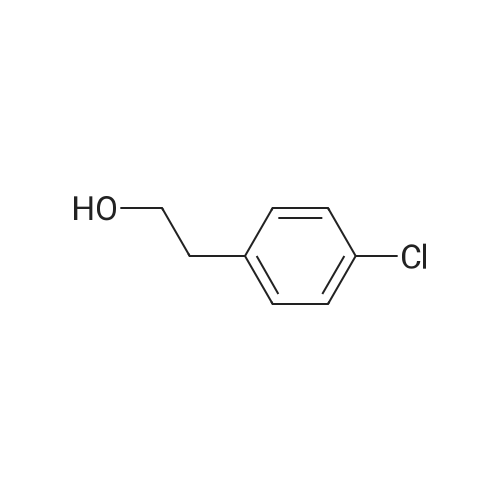
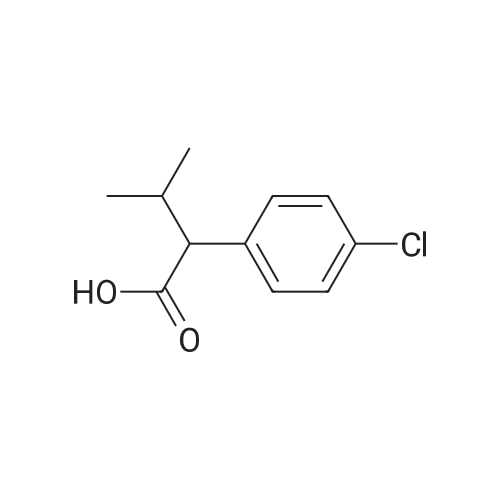
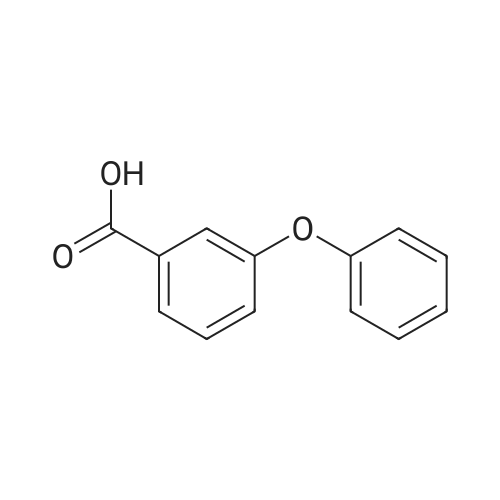
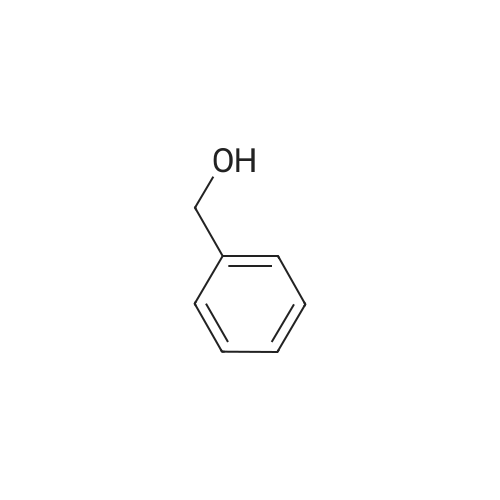
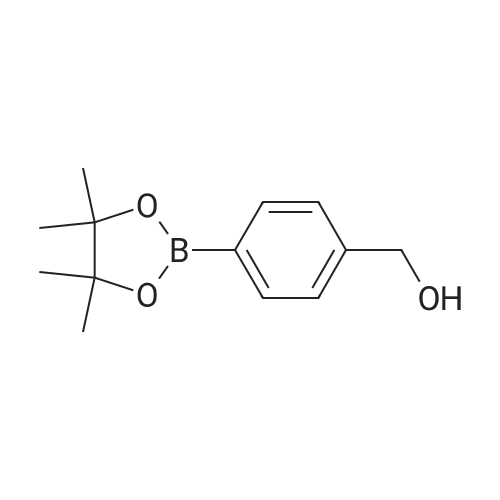
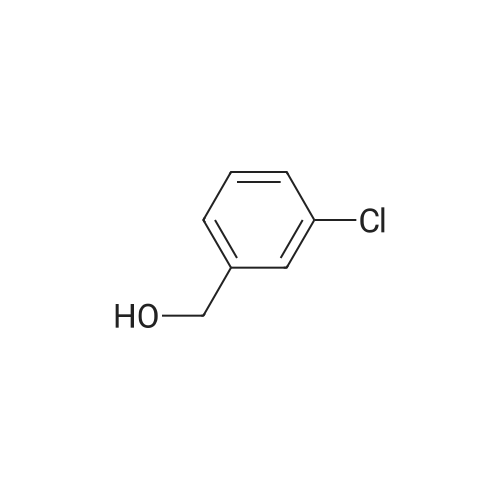
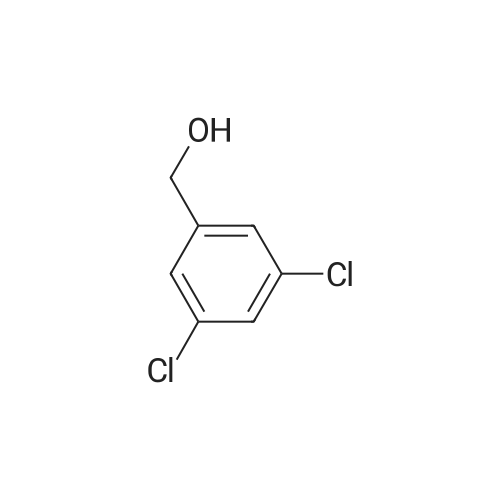
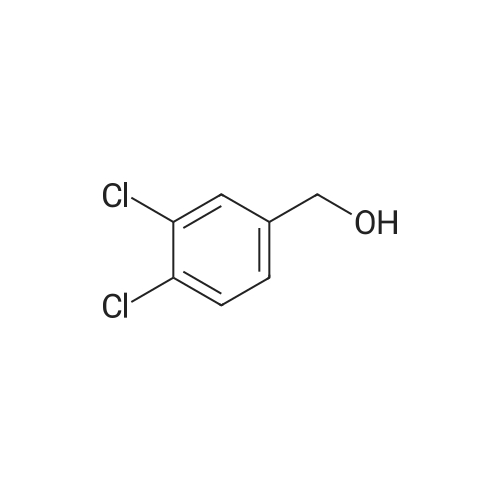
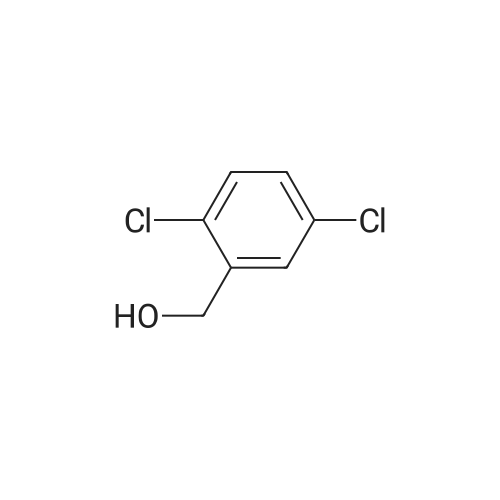
| 86% |
With C79H98Cl3IrN4P2Ru; potassium hydroxide; In 1,4-dioxane; at 110℃; for 18h;Inert atmosphere; Schlenk technique; |
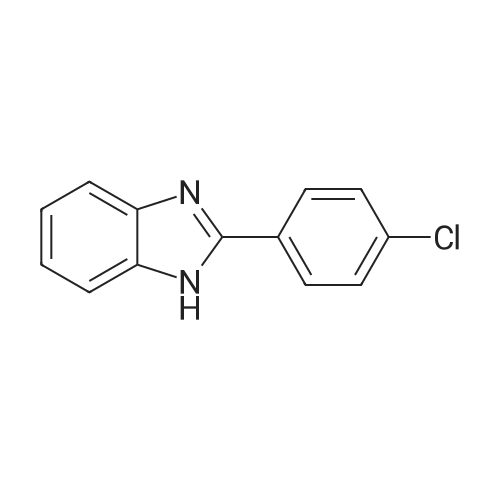
2 - chlorophenyl -1HSynthesis of benzimidazole -: in an inert gas (such as high-pure nitrogen) under the protection, to 10 ml of the reaction tubes to join Schlek 0.02mmol ruthenium iridiumdifferent nuclear ring metal compound 15, 1.0mmol O-phenylendiamine, 1.9mmol to the chlorine animal pen is mellow, 0.2mmol potassium hydroxide and 3 ml of dioxane, replace the nitrogen reaction tube 3 times, then in the oil bath for magnetic stirring under heating to 110 C, reaction reflux 18 hours. To remove the oil bath, water bath to room temperature; to responds fluid Canada 3 ml water, for 5 ml of dichloromethane extraction three times, and the combined organic phase with anhydrous MgSO4Drying 30 minutes, filtered; the filtrate using a rotary evaporator concentrated, petroleum ether/ethyl acetate to residual liquid as developing agent, silica gel thin layer chromatography separation, to obtain the pure product 2 - chlorophenyl -1H- Benzimidazole, yield 86% |
| 86% |
In acetonitrile;Irradiation; Inert atmosphere; |
The photocatalytic synthesis of benzimidazole was investigaded by using Co-TiO2 catalyst under solar light irradiation. In a typical experiment, orthophenylene diamine (0.226g, 1.66mmol) was dissolved (5ml solution of benzyl alcohol (0.213g, 1.75mmol) in acetonitrile (5ml), and 50mg of photocatalyst was added to 100ml quartz photo reactor. Prior to photoirradiation, the suspensions were magnetically stirred while the reactor was purged with nitrogen gas for 20min to generate inert atmosphere in the photo reactor. The photocatalytic reaction was carried out under solar light irradiation and the reaction mixture was magnetically stirred during the entire period (8h) of experiment. The progress of the reaction was monitored with TLC. After completion of the reaction, the photocatalyst was separated by centrifugation. The mixture was treated with ethyl acetate and then concentrated in vacuo. The residue was purified by silica-gel column chromatography with ethyl acetate/hexane (1:4) to afford the product. |
| 85% |
With Fe2(SO4)3; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; In neat (no solvent); at 110℃; for 24h;Green chemistry; |
General procedure: A mixture of 6 mmol of the alcohol or the amine and5 mmol o-phenylenediamine, o-aminophenol or o-aminothiophenol,10 mol % Fe2(SO4)3, 10 mol % TEMPO wasprepared in a 10 ml three-necked flask, and then stirred inopen air at 110 C for several hours, The reaction progresswas monitored by TLC. When the final reaction mixturecooled to room temperature, the crude products was directlypurified by column chromatography on silica gel using hexane/ethyl acetate (7:3) as eluent to afford the pure product. |
| 85% |
With anhydrous sodium carbonate; In neat (no solvent); at 120℃;Green chemistry; |
General procedure: Typically, o-phenylenediamine (1.3 mmol) or 2-aminothiophenol (1 mmol), benzyl alcohols (1 mmol), Na2CO3 (20 mol%), and Pd-NPs/Cu2(BDC)2(DABCO) (20 mg, 0.01 mol%) were added to a round-bottom flask. The reaction mixture was heated to 120 Cand stirred at for the appropriate time in air (TLC monitoring). Ethyl acetate was added to the reaction mixture and catalyst was filtered. For the purification of impure products, chromatography on silica gel was performed (EtOAc:Hep. (1:6)). The entire products characterized by melting point, CHN, 1H-NMR and13C-NMR spectroscopy. |
| 82% |
In neat (no solvent); at 135℃; for 24h;Green chemistry; |
General procedure: Reactions were performed in a magnetically stirred round bottomed flask fitted with acondenser and placed in a temperature controlled oil bath. 1,2-Diamine (2 mmol)was added to alcohol (3 mmol) and the reaction mixture was allowed to stir at 135C in an open (air) atmosphere. After disappearance of the diamine (reaction was monitored by TLC)or after the appropriate time, the reaction mixture was cooled to roomtemperature. The crude residue was further purified by column chromatography using silica gel (100-200 mesh) to afford pure products. All the products wereidentified on the basis of NMR and mass spectral data |
| 80% |
With N-hydroxyphthalimide; at 70℃; for 3h; |
General procedure: To a mixture of benzyl alcohol (1.2mmol), 1,2-phenylenediamine(1mmol) and NHPI (5mol%) in a glass test tube(10cm tall × 1cm diameter) was added TiO2/AA/Co nanocatalyst(0.06mol%) and the reaction mixture was heatedat 70C under air, visible light and solvent free conditions.The reaction progress was monitored by TLC. After 3h,ethanol (5mL) was added to the mixture and then TiO2/AA/Co nanocatalyst (solid phase) was separated by centrifugingfollowed by decantation (3 × 5mL ethanol). Desired product(liquid phase) was extracted by plate chromatography elutedwith n-hexane/EtOAc (10/2). Assignments of products weremade by 1H NMR spectral data in comparison with authenticsamples. |
| 80% |
With copper(II)-manganese(II) bimetallic complex immobilized on magnetite nanoparticles; air; In neat (no solvent); at 70℃; for 2h;Green chemistry; |
General procedure: The one-pot synthesis of benzimidazole carried out in around-bottom flask with benzyl alcohol (1.2mmol) and ortho phenylenediamine (1.0mmol) using Fe3O4Cu-Mn catalyst (0.008g, 0.280mol% Cu, 0.078mol% Mn), under solvent-free and aerobic conditions at 70C. The reaction mixture was monitored with TLC. Upon reaction completion, the catalyst was magnetically separated, then the product was extracted to dichloromethane (3 × 5mL).The organic layers were combined, dried over Na2SO4 and purified by silica-gel column chromatography (n-hexane:EtOAc = 10: 5). |
| 73% |
With 3-nitropyridine; sodium tertiary butoxide; In dimethyl sulfoxide; at 110℃; for 16h; |
General procedure: A 25 mL RB was charged with a mixture of 2-aminophenols 1a-h (1.0 mmol) or 1-amino-2-naphthol 4a (1.0 mmol) or 2-aminothiophenol 6a (1.0 mmol) or o-phenylenediamine 7a (1.0 mmol) and benzyl alcohols 2a-e, g-j or cinnamyl alcohol 2f (1.0 mmol) along with 3-nitropyridine (0.1 mmol, 12.4 mg), NaOtBu (1.0 mmol, 96 mg) and DMSO (2 mL). The RB was loosely fitted with septum and then heated at 110 C for 16 h. After completion of the reaction, the mixture was diluted with hot ethyl acetate (20 mL) and water (40 mL) and extracted with ethyl acetate (3 10 mL). The combined organic layer was washed with brine (2 10 mL) and dried over anhydrous Na2SO4. Solvent was removed under reduced pressure and the remaining residue was purified by flash chromatography over silica gel using hexane / ethyl acetate = 9:1 (v/v) as an eluent to obtain the desired products benzoxazoles 3a-v, naphthoxazoles 5a-f, benzothiazoles 8a-f and benzimidazoles 9a-c in high yields. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping