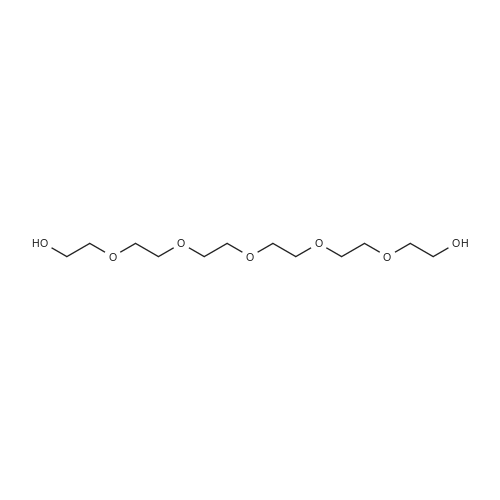
| 90% |
With triethylamine; In dichloromethane; for 0.5h; |
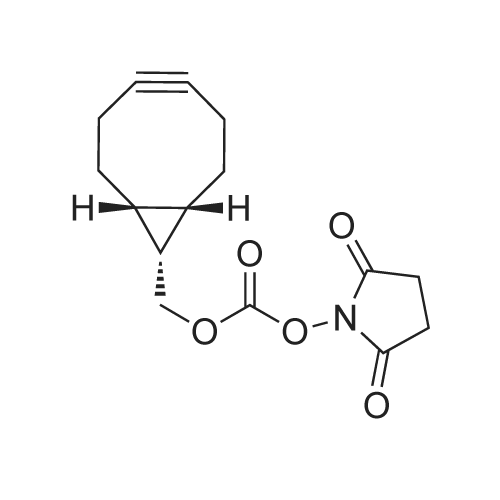
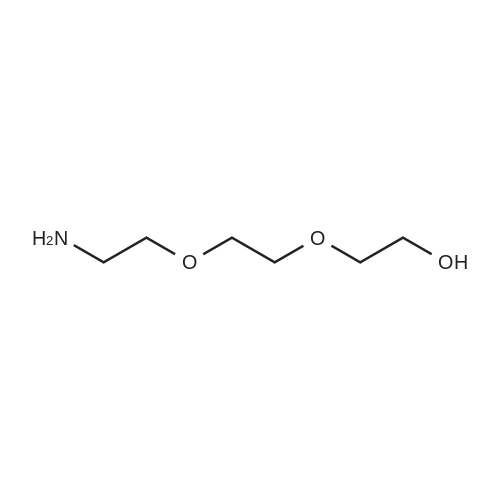
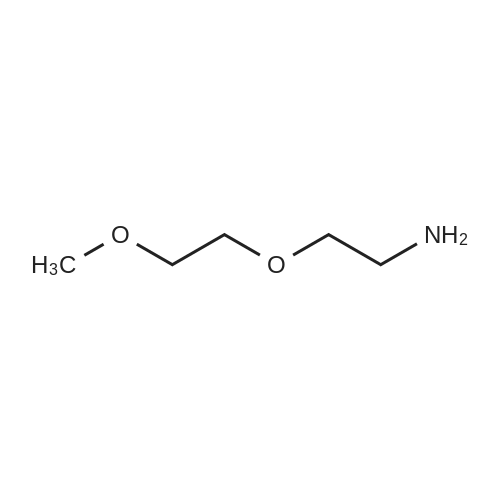
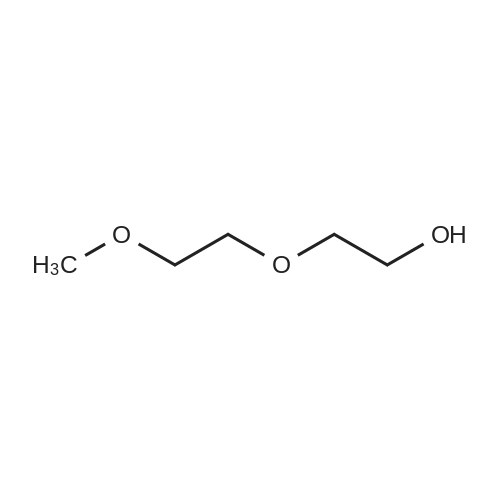
To a solution of 2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethanol (256 mg, 1.32 mmol) in DCM (30 mL) were added BCN-OSu (351 mg, 1.20 mmol) and Et3N (502 μΙ_, 364 mg, 3.60 mmol). The resulting solution was stirred for 30 min and washed with a saturated aqueous solution of NH4CI. After separation, the aqueous phase was extracted with DCM (30 mL). The combined organic phases were dried (Na2S04) and concentrated. The residue was purified with column chromatography (MeOH in DCM 0→ 10%). The product was obtained as a colourless oil (397 mg, 1.07 mmol, 90%). H NMR (400 MHz, CDCI3) δ (ppm) 5.93 (bs, 1 H), 4.14 (d, J = 8.1 Hz, 2H), 3.77-3.69 (m, 4H), 3.68-3.59 (m, 8H), 3.58-3.52 (m, 2H), 3.42-3.32 (m, 2H), 2.34-2.16 (m, 6H), 1.66-1.51 (m, 2H), 1 .36 (quintet, J = 8.7 Hz, 1 H), 1.00-0.85 (m, 2H). |
| 67% |
With triethylamine; for 1.33333h; |
To a solution of 2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethanol (0.50 g, 2.6 mmol) was added BCN-OSu (29-1 , 0.69 g, 2.3 mmol) and Et3N (0.97 ml_, 0.70 g, 7.0 mmol). The resulting mixture was mixture was stirred for 80 min and saturated aqueous NH4CI (60 ml_) was added. After separation, the aqueous phase was extracted with DCM (60 ml_). The combined organic layers were dried (Na2SC>4) and concentrated. The residue was purified with silica gel chromatography (DCM 10% MeOH in DCM), which yielded 578 mg (1.56 mmol, 67%) of the desired product. 1 H NMR (400 MHz, CDCIs) d (ppm) 5.98-5.85 (bs, 1 H), 4.14 (d, J = 8.0 Hz, 2H), 3.78-3.59 (m, 12H), 3.58-3.52 (m, 2H), 3.42-3.32 (m, 2H), 3.19-3.06 (bs, 1 H), 2.35-2.16 (m, 6H), 1 .70-1.50 (m, 2H), 1.36 (quintet, J = 8.6 Hz, 1 H), 0.99-0.87 (m, 2H). |
|
With triethylamine; In acetonitrile; for 1h; |
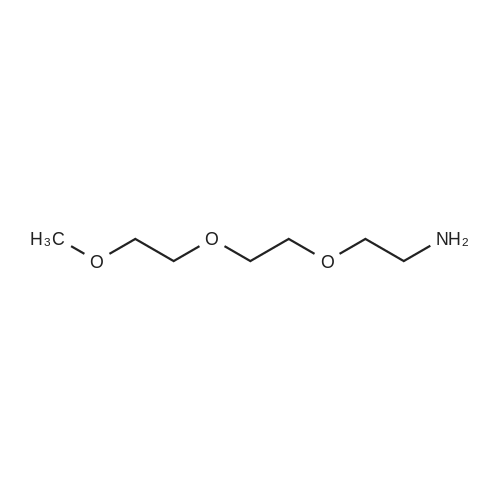
To 300 mg of <strong>[1426827-79-3]BCN-NHS</strong> (1.03 mmol) 10 ml of acetonitrile were added followed by addition of 1.0 ml triethylamine. To reaction solution first 0.3 ml of OHPEG 4-NH2 was added and reaction was stirred for half hour before it was tested byTLC reaction was not complete. Then additional 0.2 ml of OH-PEG4-NH2 were added to total 0.5 ml of OH-PEG4-NH2 (2.83 mmol) and reaction was stirred for half hour till completion as was observed by TLC (TLC mobile phase: 90% Chloroform, 10% Methanol; Staining PMA). Reaction solution was evaporated under reduced pressure to dryness by rotovapor followed by purification by silica column. The reactionsolution was eluted with 5% MeOH: 95% dichloromethane. The pure fractions were evaporated to dryness by rotovapor. The obtained product - 200 mg (53%) was tested by TLC. According to TLC the purity was about 90% therefore the product was used as is the next step/steps. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping