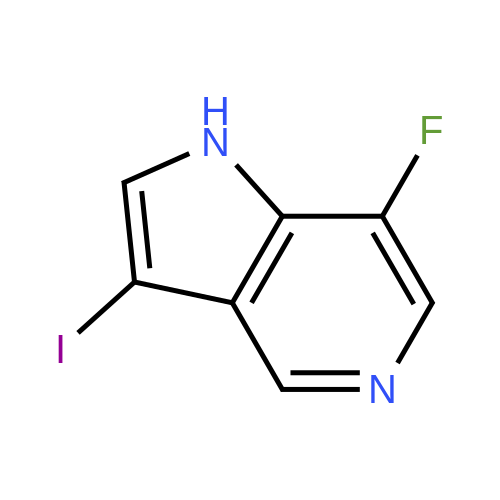
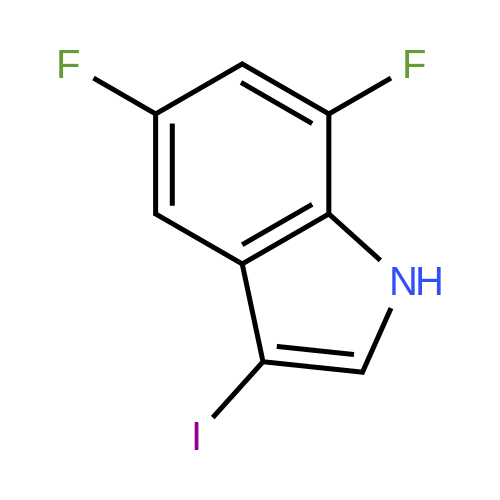
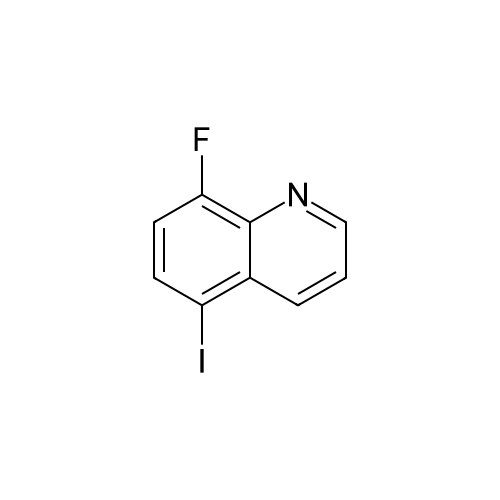
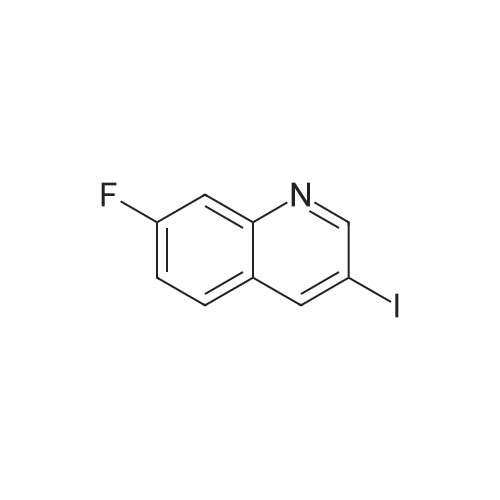
| 33% |
With potassium carbonate; N,N`-dimethylethylenediamine;copper(l) iodide; In dimethyl sulfoxide; at 100℃; for 8h; |
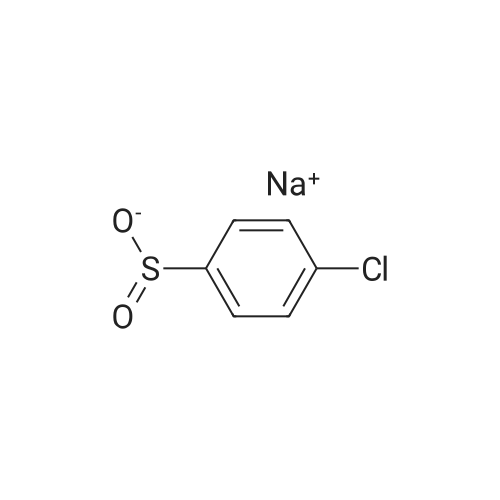
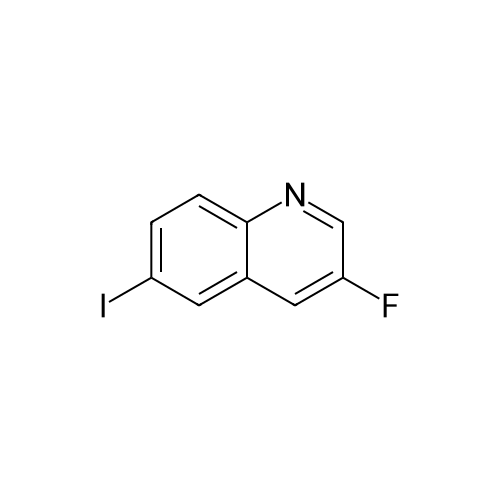
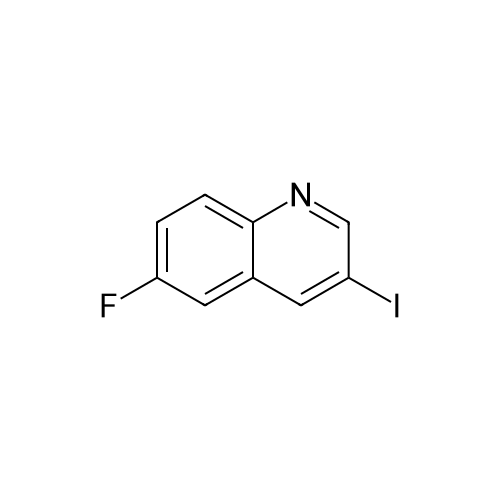
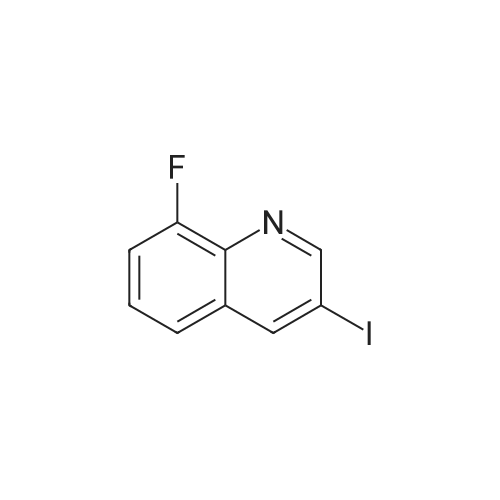
Description 3 3-[(4-Chlorophenyl)sulfonyl]-8-fluoroquinoline (D3) EPO <DP n="19"/>A mixture of 8-fluoro-3-iodoquinoline (D1 ) (750 mg, 2.75 mmol), sodium 4- chlorobenzenesulfinate (1.1 g, 5.5 mmol), copper (I) iodide ( 52 mg, 0.275 mmol) and potassium carbonate (380 mg, 2.75 mmol) was treated with λ/./V-di methyl- 1 ,2- ethanediamine (49 mg, 0.55 mmol) and anhydrous dimethylsulphoxide (4 ml). The mixture was stirred at 1000C under argon for 8 hr, and cooled to 200C. The reaction mixture was diluted with water (60 ml) and extracted with ethyl acetate (3 x 40 ml). The organic extracts were combined, washed with water (60 ml) and brine (60 ml), dried over magnesium sulphate, and evaporated to dryness. The residue was dissolved in a 1 :1 mixture of dimethylsulphoxide and acetonitrile and purified by mass-directed auto- preparative chromatography using 10 minute gradients containing water and between 50% and 99% acetonitrile with 0.1% formic acid. Product fractions were collected and evaporated to yield the title compound as a white solid (295 mg, 33%). δH (CDCI3, 400MHz) 7.51-7.69 (4H, m), 7.79 (1 H, d, J = 8 Hz), 7.96-7.99 (2H, m), 8.84 (1 H, d, J = 2 Hz), 9.30 (1 H, d, J = 2 Hz) Mass spectrum: C15H9CIFNO2S requires 321 ; found 322 (MH+) |
| 33% |
With copper(l) iodide; potassium carbonate; N,N-dimethylethylenediamine; In dimethyl sulfoxide; at 100℃; for 8h; |
A mixture of 8-fluoro-3-iodoquinoline (750 mg, 2.75 mmol), sodium 4- chlorobenzenesulfinate (1.1 g, 5.5 mmol), copper (I) iodide ( 52 mg, 0.275 mmol) and potassium carbonate (380 mg, 2.75 mmol) was treated with λ^, λ^-dimethyl-l,2-ethanediamine (49 mg, 0.55 mmol) and anhydrous dimethylsulphoxide (4 ml). The mixture was stirred at 1000C under argon for 8 hr, and cooled to 200C. The reaction mixture was diluted with water <n="215"/>(60 ml) and extracted with ethyl acetate (3 x 40 ml). The organic extracts were combined, washed with water (60 ml) and brine (60 ml), dried over magnesium sulphate, and evaporated to dryness. The residue was dissolved in a 1 :1 mixture of dimethylsulphoxide and acetonitrile and purified by mass-directed auto -preparative chromatography using 10 minute gradients containing water and between 50% and 99% acetonitrile with 0.1% formic acid. Product fractions were collected and evaporated to yield the title compound as a white solid (295 mg,δH (CDCl3, 400MHz) 7.51-7.69 (4H, m), 7.79 (IH, d, J = 8 Hz), 7.96-7.99 (2H, m), 8.84 (IH, d, J = 2 Hz), 9.30 (IH, d, J = 2 Hz)Mass spectrum: C15H9ClFNO2S requires 321; found 322 (MH+) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping