| 88% |
With 1,4-diaza-bicyclo[2.2.2]octane; N-ethyl-N,N-diisopropylamine; In dichloromethane; at 35 - 40℃; for 0.5h;Product distribution / selectivity; |
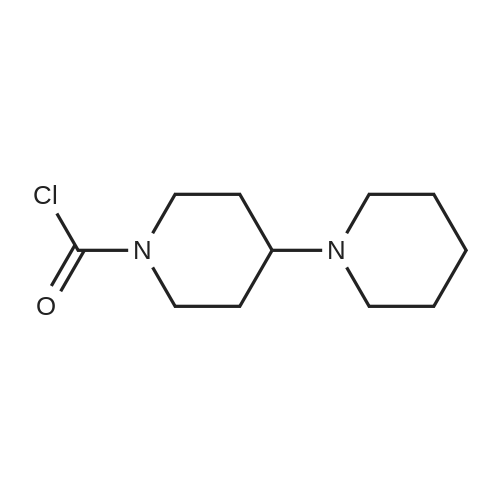
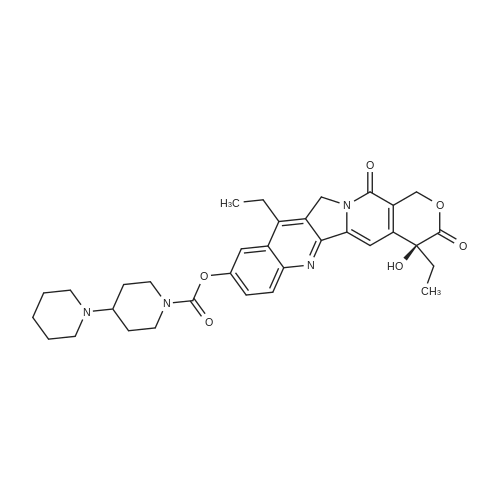
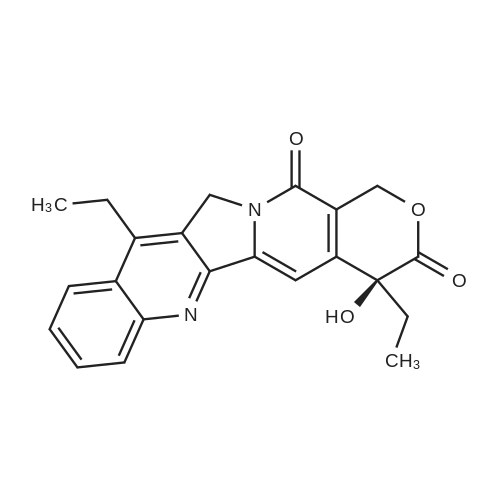
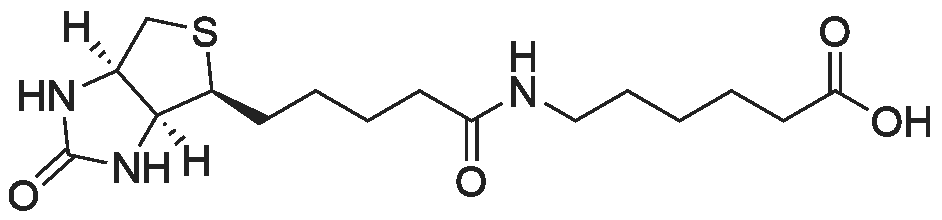
EXAMPLE 1 A mixture of 25.02 g (0.0637 mol) 7-ethyl-10-hydroxycamptothecin, 18.72 g (0.07 mmol) of 4-piperidinopiperidinecarbonylchloride, 0.99 g (6.4 mmol) DABCO and 400 ml dichloromethane is treated with 18.93 g (0.146 mol) N,N-Diisopropylethylamine (DIEA) at 35 to 40° C. After 0.5 h complete conversion (>99percent), is observed. Subsequently, the organic layer is washed 3 times with NH4Cl-solution (27percent), KHCO3-solution (25percent) and NaCl-solution (26percent). Active charcoal is added, and the suspension is warmed to reflux for at least 1 h. Charcoal is filtered off and subsequently 800 ml t-Butylmethylether (t-BME) is added within 30 min at reflux. The mixture is cooled to 35-40° C. (precipitation of the product) and stirred for at least 1 h at 35-40° C. The suspension is cooled to 0-5° C., stirred for at least 1 additional hour and subsequently filtered off and dried in vacuo. The crude product (Irinotecan free base) is crystallized from 2-methoxyethanol. Yield: 32.9 g (88percent of theory) Appearance: yellow, crystalline powder |
| 63.5% |
With sodium hydrogencarbonate; In pyridine; chloroform; |
EXAMPLE 28 7-Ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin 7-Ethyl-10-hydroxycamptothecin (790 mg, 2.01 mmol) and 1-chlorocarbonyl-4-piperidinopiperidine (910 mg, 3.95 mmol) were dissolved in anhydrous pyridine (50 ml), and the mixture was stirred for 1 hour at room temperature. The reaction mixture was evaporated to dryness in vacuo and the residue was dissolved in CHCl3 (200 ml). The solution was washed successively with a 7percent aqueous solution of NaHCO3 (200 ml), a saturated aqueous solution NaCl, and the CHCl3 layer was dried with MgSO4, filtered, and evaporated in vacuo. The residual material was decolorized by passing it through a short silica gel column whereby 1.11 g (94.8percent in yield) of the title compound was obtained as a pale yellow mass, which was recrystallized from ethanol (ca. 60 ml) to give colorless needles (750 mg, 63.5percent in yield). |
| 63.5% |
With pyridine; at 20℃; for 1h; |
790 mg (2.01 mmol) of 7-ethyl-10-hydroxycamptothecin (SN-38)And 910 mg (3.9 5 mmol) of 1-chlorocarbonyl-4-piperidinopiperidine hydrochloride,Was mixed with anhydrous pyridine (50 mL).The dissolved mixture was stirred at ambient temperature for 1 hour.The mixture was concentrated under reduced pressure.The concentrated residue was dried under reduced pressure.The dried residue was dissolved in chloroform (200 mL).The dissolved solution was washed successively with 7percent sodium bicarbonate (NaHCO 3) (200 mL) and saturated brine,Dry over sodium sulfate,And concentrated using a vacuum condenser.After concentration,The product was refined by liquid chromatography,Recrystallization with ethanol (60 mL) gave 750 mg (yield: 63.5percent) of the title compound. |
|
|
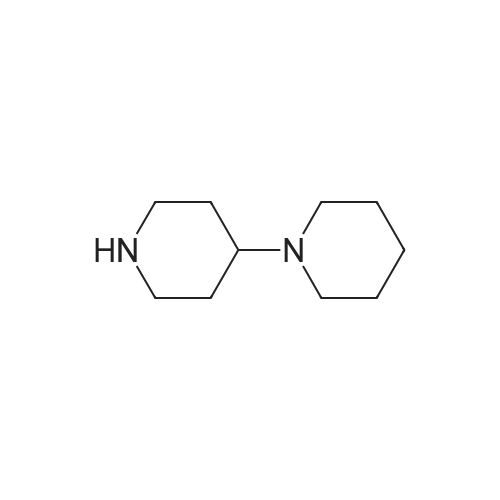
The compound 4 was dissolved in pyridine and reacted with 4-piperidinopiperidinecarbamyl chloride dissolved in DCM. The DCM and pyridine are removed by distillation and the crude mixture was worked up by dissolving the crude mixture in DCM and treating with saturated aqueous sodium bicarbonate solution, collecting the organic phase and purifying using column chromatography to afford pure compound 2. |
|
With pyridine; at 40℃; for 2h;Product distribution / selectivity; |
EXAMPLE 4 Preparation of Irinotecan 4-piperidinopiperidine carbamoyl chloride obtained from Example 3 was dissolved in 3.24 L of pyridine, followed by the addition of 54 g (0.137 moles) of 7-ethyl 10-hydroxy camptothecin, prepared according to Example 2. The mixture was heated to 40° C. with simultaneous stirring for 2 hours under a nitrogen atmosphere. The reaction mixture was cooled to about 27° C.; then filtered through a celite bed and the celite was washed with 200 ml of pyridine. The filtrate was concentrated to get 250 ml solvent remaining at 50° C. under a vacuum of 650 mm Hg. The obtained residue was dissolved in 1 L of water and filtered through celite. The obtained filtrate was washed with 500 ml of n-heptane and with 2*500 ml of ethyl acetate. The aqueous layer was concentrated at 50° C. to a 250 ml volume remained and then codistilled with 2*1 L of isopropyl alcohol at 50° C. to get 500 ml solvent remaining. The obtained suspension was allowed to cool to room temperature and then filtered. The filtered solid was washed with 100 ml of isopropyl alcohol and finally subjected to drying at 70° C. for 3 hours under a vacuum of 650 mm Hg to afford 69.6 g of crude irinotecan. Purity: 94.7percent by HPLC. EXAMPLE 5; Preparation of Irinotecan; 18 g of 4-piperidinopiperidine carbamoyl chloride, obtained according to Example 3, was dissolved in 637 ml of pyridine, followed by the addition of 10 g of 7-ethyl 10-hydroxy camptothecin, prepared according to Example 2. The mixture was heated to 40° C. with simultaneous stirring for 2 hours under a nitrogen atmosphere. The above reaction mixture was cooled to about 27° C., and then filtered through a celite bed. The filtrate was concentrated at 50° C. under a vacuum of 650 mm Hg to a volume of 40 ml. 185 ml of n-heptane was added to the residue and concentrated at 50° C. under vacuum of 580 mm Hg to a volume of 50 ml. The above step was repeated two more times and then 185 ml of water was charged to the resultant residue. Adjusted the pH of the reaction mixture to 5.7 with 1 ml of acetic acid and then separated the two layers, and washed the aqueous layer with 2.x.92.5 ml of ethyl acetate. The obtained aqueous layer was concentrated at 45° C. under a vacuum of 590 mm Hg to a volume of 50 ml. 185 ml of isopropyl alcohol was charged to the residue and concentrated to a volume of 50 ml. Again 185 ml of isopropyl alcohol was charged to the above obtained residue and concentrated at 45° C. under a vacuum of 580 mm Hg to a volume of 50 ml. The suspension was cooled to 27° C. and stirred for 1 hour. The obtained suspension was filtered and washed with 20 ml of isopropyl alcohol and the solid dried at 65° C. under a vacuum of 580 mm Hg for 4 hours to afford 11.5 g of the title compound. Purity: 96.47percent by HPLC. |
|
With pyridine; for 6 - 12h; |
Example - 5; Preparation of Irinotecan (IRT-5); Dissolving 7-ethyl-10-hydroxycamptothecin (50g) obtained in example 4(c) in pyridine (200 ml) under stirring at room temperature. Adding to it a solution of 1-chlorocarbonyl-4-piperidino-piperidine base (90g) in pyridine (2000 ml) and stirring for further 6 hours to 12 hours. Distilling off pyridine completely under vacuum at a temperature preferably below 45°C, cool the residue to room temperature, dissolving in chloroform (2500 ml), washing the chloroform solution with an aqueous sodium bicarbonate, followed by water. Separating chloroform layer, removing chloroform completely under vacuum, adding n-hexane filtering, drying the solid, purifying by column chromatography to obtain purified Irinotecan (IRT-5, 30g) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +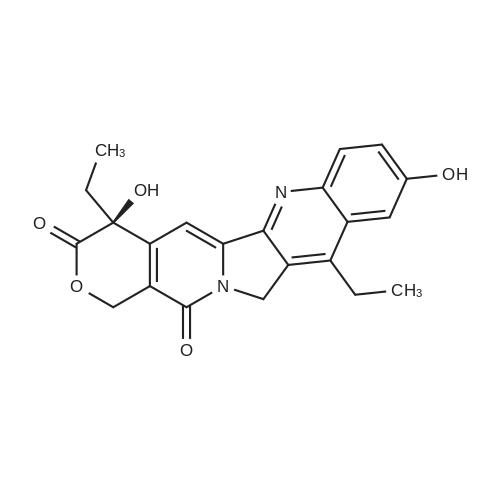

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping