| 40% |
With bromine; In N,N-dimethyl-formamide; at 20℃; for 4h; |
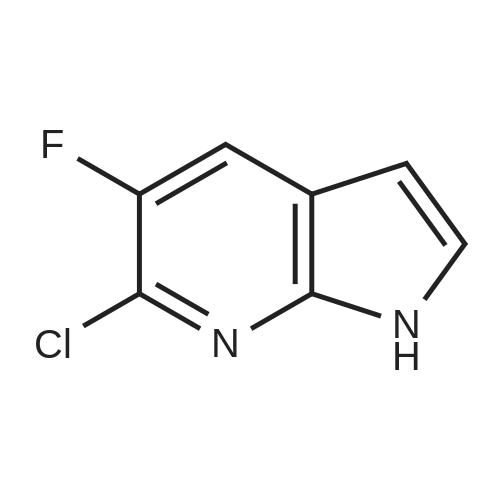
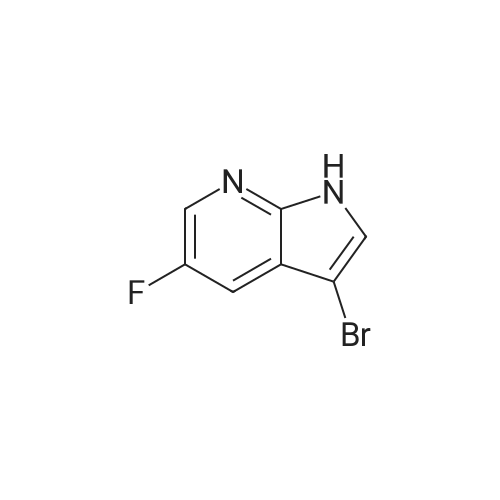
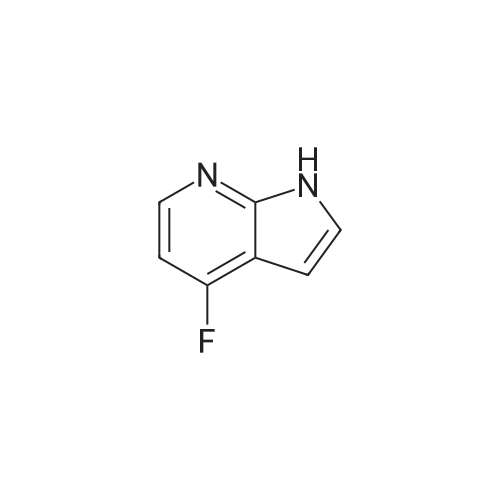
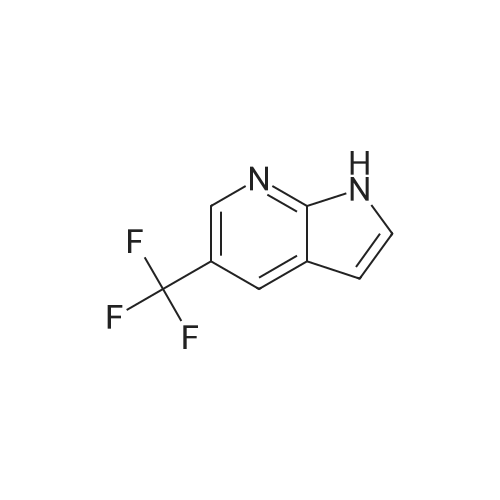
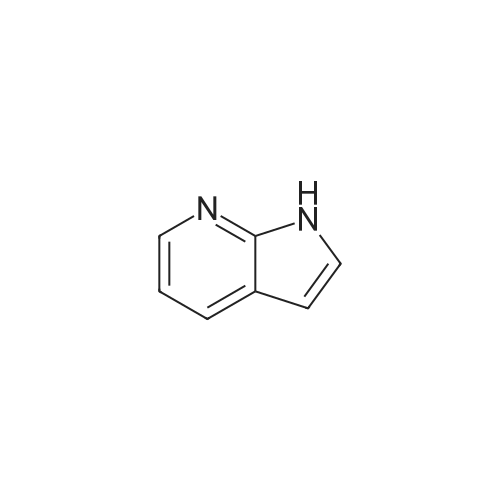
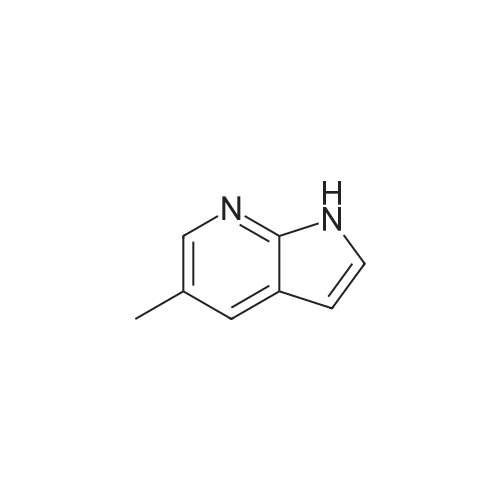
To the solution of 5-fluoro-1H-pyrrolo[2,3-b]pyridine (1 g, 7.34 mmol) in DMF (10 mL) was added bromine (0.75 mL, 14.5 mmol). The mixture was stirred at it for 4 hours. The reaction mixture was quenched with saturated aqueous sodium thiosulfate (100 mL). The resulting mixture was extracted with ethyl acetate (100 mL x 2). The combined organic layers were washed with saturated brine (100 mL x 3), dried over ahydrous sodium sulfate and filtered. The filtrate was concentrated in vacuo, and the residue was purified by silica gel column chromatography (PE/EtOAc (v/v) = 4/1) to give the title compound as yellow powder (0.6 g, 40%).MS (ESI, pos. ion) m/z: 216.9 [M+H]+1H NMR (400 MHz, DMSO-d6) ? (ppm): 12.23 (s, 1H), 8.35-8.21 (m, 1H), 7.81 (d, J = 2.7 Hz, 1H), 7.71 (dd, J = 8.9, 2.6 Hz, 1H). |
| 40% |
With bromine; In N,N-dimethyl-formamide; at 20℃; for 4h; |
To a solution of 5-fluoro-1H-pyrrolo[2,3-b]pyridine (1.0 g, 7.34 mmol) in DMF (10 mL) was added bromine (0.75 mL, 14.5 mmol) . The reaction mixture was stirred at r. t. for 4 h. A saturated aqueous Na2S2O3 solution (100 mL) was added to quench the reaction, and the resulting mixture was extracted with EtOAc (100 mL × 2) . The combined organic phases were washed with saturated brine (100 mL × 3) , dried over anhydrous Na2SO4, filtered, and the filtrate was concentrated in vacuo. The residue was purified by silica gel chromatograph (PE/EtOAc (v/v) 4/1) to give the tilte compound as a yellow solid (0.6 g, 40 %). MS (ESI, pos. ion) m/z: 216.9 [M+H]+ 1H NMR (400 MHz, DMSO-d6) δ (ppm) : 12.23 (s, 1H) , 8.35-8.21 (m, 1H) , 7.81 (d, J 2.7 Hz, 1H) , 7.71 (dd, J 8.9, 2.6 Hz, 1H). |
| 40% |
With bromine; at 20℃; for 4h; |
5-Fluoro-1H-pyrrolo[2,3-b]pyridine (1 g, 7.34 mmol) was dissolved in DMF (10 mL)Then, bromine (0.75 mL, 14.5 mmol) was added dropwise thereto, and the reaction was stirred at room temperature for 4 hours.Quenched with saturated aqueous sodium thiosulfate solution (100 mL)The reaction mixture was extracted with ethyl acetate (100 mL×2).The combined organic phases were washed with brine (100 mL×3)Filtered, concentrated under reduced pressure,The residue was subjected to silica gel column chromatography(PE/EtOAc (v/v) = 4/1) purified,The title compound was obtained as a yellow powder (0.6 g, 40%). |
| 40% |
With bromine; In N,N-dimethyl-formamide; at 20℃; for 4h; |
5-Fluoro-1H-pyrrolo[2,3-b]pyridine (1 g, 7.34 mmol) was dissolved in DMF (10 mL)Then bromine (0.75 mL, 14.5 mmol) was added dropwise thereto.The reaction was stirred at room temperature for 4 hours.The reaction was quenched by the addition of saturated aqueous sodium thiosulfate (100 mL).The resulting mixture was extracted with ethyl acetate (100 mL X 2).The combined organic phases were washed with saturated brine (100 mL×3).Dry over anhydrous sodium sulfate, filter,Concentrated under reduced pressure, and the residue was applied to silica gel column chromatography(Petroleum ether / ethyl acetate (ν / ν) = 4 / 1) purification,The title compound was obtained as a yellow powder (0.6 g, 40%). |
|
With N-Bromosuccinimide; In dichloromethane; at 20℃; for 19h; |
Preparation of compound 2: N-bromosuccinimide (NBS, 5 · 29 g, 29 · 7 mmol) was added to a solution of compound 1 (4.50 g, 33 mmol) in dichloromethane (100 mL) and react at room temperature 19 hour,After the reaction, a saturated sodium hydrogen sulfite solution (200 ml) was added, and the mixture was separated.The organic layer was washed with 20% sodium hydroxide solution.Dry over anhydrous sodium sulfate, filter,The filtrate was concentrated in vacuo to give a crude product4.62g. |
|
With N-Bromosuccinimide; In dichloromethane; at 20℃; for 19h; |
N-bromosuccinimide (NBS, 5.29 g, 29.7 mmol) was added to Compound 6(4.50 g, 33 mmol) in dichloromethane (100 mL) and reacted at room temperature for 19 hours,After the reaction was completed, a saturated sodium bisulfite solution (200 mL) was added.The layers were separated, and the organic layer was washed with 20% sodium hydroxide solution and dried over anhydrous sodium sulfate.Filtration and concentration of the filtrate in vacuo gave the crude product4.62g. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping