Alternatived Products of [ 863971-53-3 ]
Product Details of [ 863971-53-3 ]
| CAS No. : | 863971-53-3 |
MDL No. : | MFCD22200278 |
| Formula : |
C40H42N6O10
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
766.80
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 863971-53-3 ]
Application In Synthesis of [ 863971-53-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 863971-53-3 ]
- 1
-
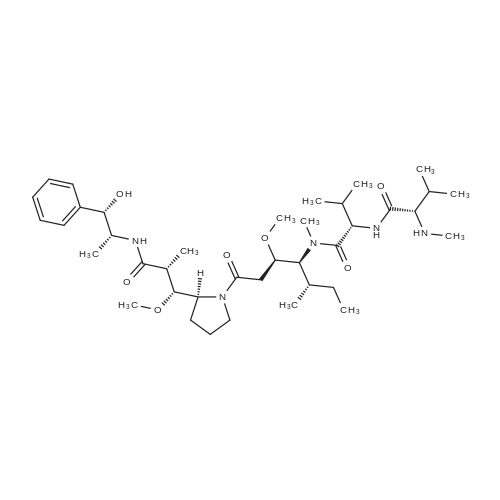 [ 474645-27-7 ]
[ 474645-27-7 ]

-
 [ 863971-53-3 ]
[ 863971-53-3 ]

-
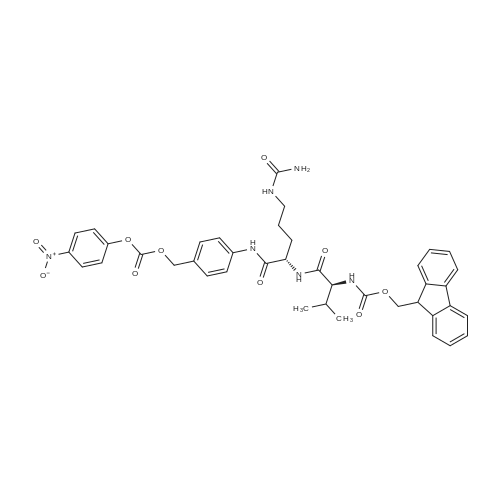
Fmoc-VC-PAB-MMAE
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 68% |
With benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 24h; |
A 100 ml RB flask was charged with <strong>[474645-27-7]monomethyl auristatin E</strong>(MMAE) (200 mg, 0.279 mmcl), 1-hydroxybenzotriazole (23.34 mg, 0.173 mmol) and Fmoc-Val-Cit-PAB-pnp (269 mg, 0.351 mmol). N,NDimethylformamide (8 ml) was added then the mixture was stirred at rt upon which N,N-diisopropylethylamine (0.12 1 ml, 0.696 mmol) was slowly added via a syringe. After 1 day at rt, LCMS showed almost completion. The solvent was evaporated under high vacuum. The crude was dissolved in DMF, loaded on celite and dried. It was purified by Isco (12 g column, eluent: EtOAc/Hexanes:0-100% then 100% then DCM/MeOH: 0-20% then 20%) to give the title compound 4-7 as an off-white solid (256 mg, 68% yield). LCMS [M+H] 1346. |
| 68% |
With benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; |
A 100 ml RB flask was charged with <strong>[474645-27-7]monomethyl auristatin E</strong> (MMAE) (200 mg,0.279 mmol),1-hydroxybenzotriazole(23.34 mg,0.173 mmol) and Fmoc-Val-Cit-PAB-pnp (269 mg,0.351 mmol). N,N-Dimethylformamide (8 ml) was added then the mixture was stirred at rt upon which A/,//-diisopropylethylamine (0.121 ml,0.696 mmol) was slowly added via a syringe. After 1 day at rt,LCMS showed almost completion. The solvent was evaporated under high vacuum. The crude was dissolved in a small volume of DMF,loaded on celite and dried. It was purified by Isco (12 g silica column,eluent: EtOAc/Hexanes: 0-100% then 100% followed by DCM/MeOH: 0-20% then 20%) to give the title compound as an off-white solid (256 mg,68% yield). LCMS [M+H]+ 1346. |
|
With pyridine; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 24h;Inert atmosphere; |
To a solution of MMAE (0.15 g, 0.18 mmol) and Fmoc-Val-Cit-PAB-PNP 13 (0.152g, 0.19 mmol) in DMF (1.5 ml), was added HOBt (58 mg, 0.36 mmol), pyridine (0.12ml) and DIPEA (31 p1). The reaction mixture was stirred under N2 atmosphere at room temperature for 24h. Solvents were evaporated in vacuo and the residue triturated with ethyl acetate. The resulting solid was filtered, washed with ethyl acetate, dried to give 79. LC-MS ESI m/z 1367.7 [M+Na] This was used directlywithout further purification. |
|
With benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 25 - 30℃; for 16h; |
To a 25 mL-round-bottom flask was added Fmocvc-PAB-PNP (2a) (0.33 g, 0.43 mmol) and anhydrous DMF (5 mL). To the solution were added MMAE (la, 0.21 g, 0.21 mmol), HOBt (38 mg, 0.28 mmol) and DIPEA (74 mg, 0.57 mmol) were added at 25 C. successively. The mixture was stirred at 25-30 C. for 16 hours until MMAE was totally consumed according to LCMS. The reaction mixture was filtered through membrane and the filtrate was purified by prep-HPLC (method A) to give Fmoc-3a as a white solid, which was dissolved in DMF (5 mL). To the solution was added diethylamine (0.3 mL). The reaction mixture was stirred at 30 C. for an hour until Fmoc was removed according to LCMS. The reaction mixture was filtered through membrane and the filtrate was purified by prepHPLC (method A) to give vcPAB-MMAE (3a) (0.20 g, 58% yield) as a white solid. ESI mlz: 562.5 (M/2+H). |

Reference:
[1]Organic and Biomolecular Chemistry,2016,vol. 14,p. 9501 - 9518
[2]Patent: WO2019/119141,2019,A1 .Location in patent: Paragraph 00212; 00277; 00278
[3]Patent: WO2019/109188,2019,A1 .Location in patent: Paragraph 00308; 00309
[4]Patent: WO2016/46574,2016,A1 .Location in patent: Page/Page column 144
[5]Patent: US2018/333504,2018,A1 .Location in patent: Paragraph 0373
- 2
-
 [ 474645-27-7 ]
[ 474645-27-7 ]

-
 [ 863971-53-3 ]
[ 863971-53-3 ]

-
{4-[(2S)-2-[(2S)-2-amino-3-methylbutanamido]-5-(carbamoylamino)pentanamido]phenyl}methyl N-[(1S)-1-[(1S)-1-[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-2-[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]carbamoyl}-1-methoxy-2-methylethyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl](methyl)carbamoyl}-2-methylpropyl]carbamoyl}-2-methylpropyl]-N-methylcarbamate
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







