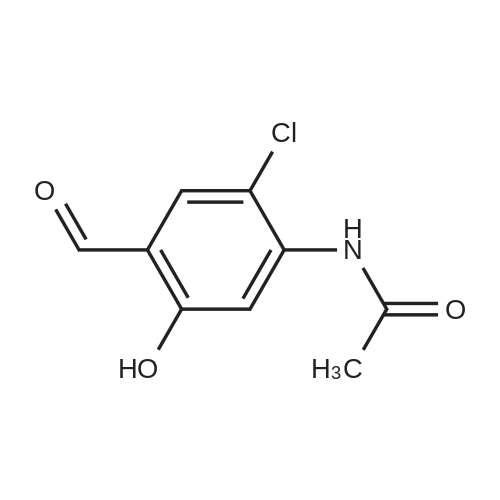
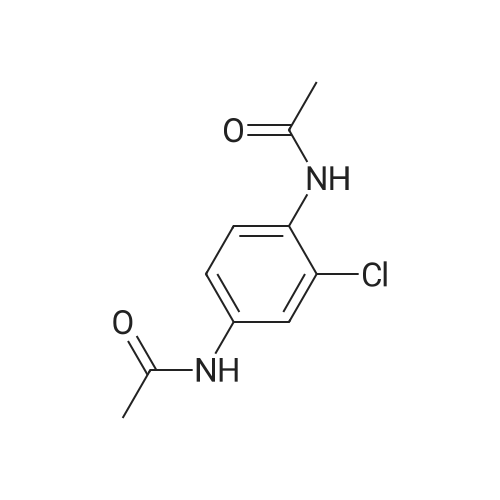
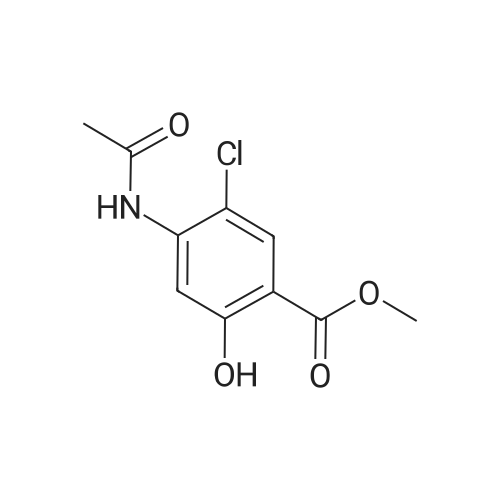
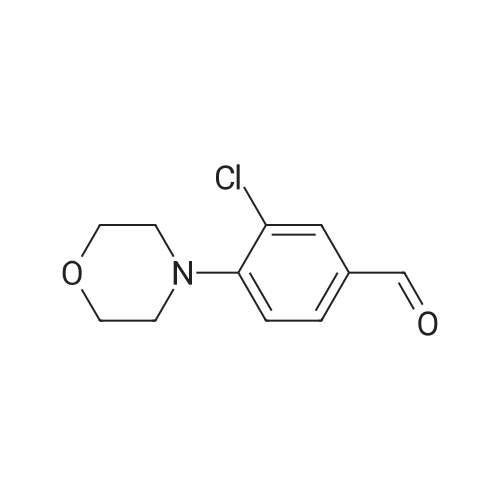
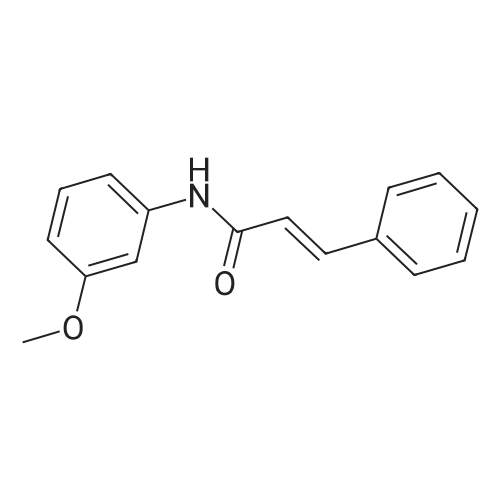
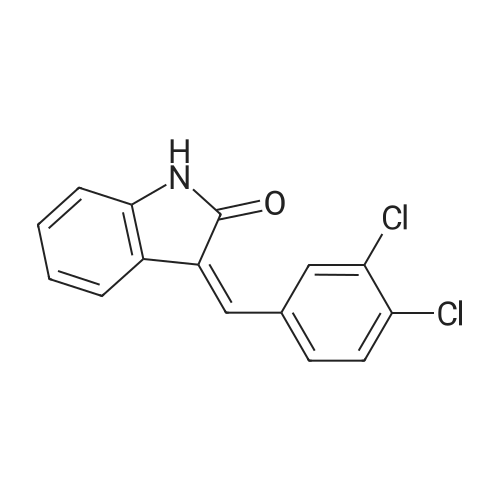
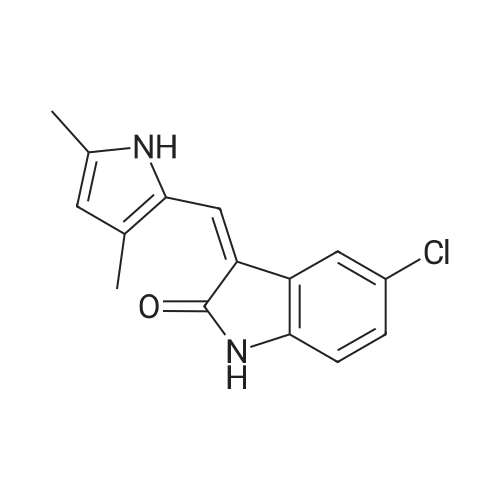
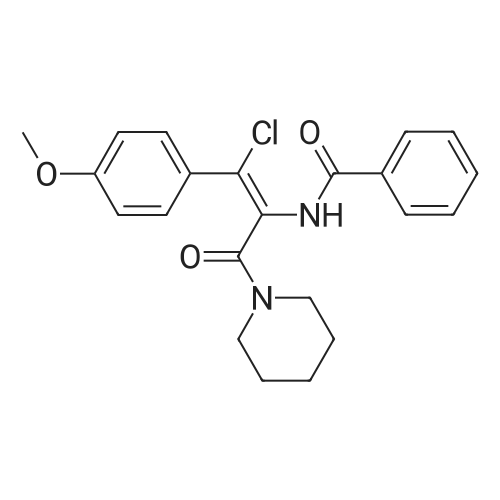
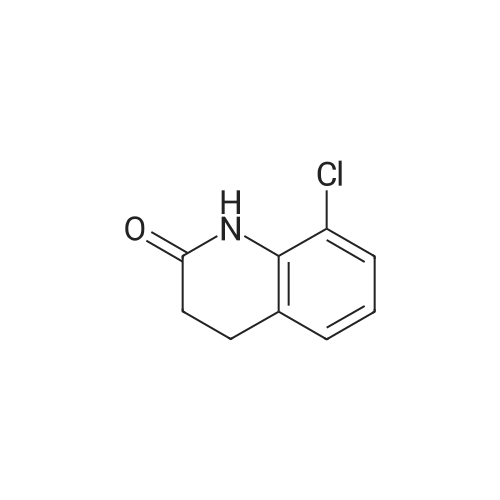
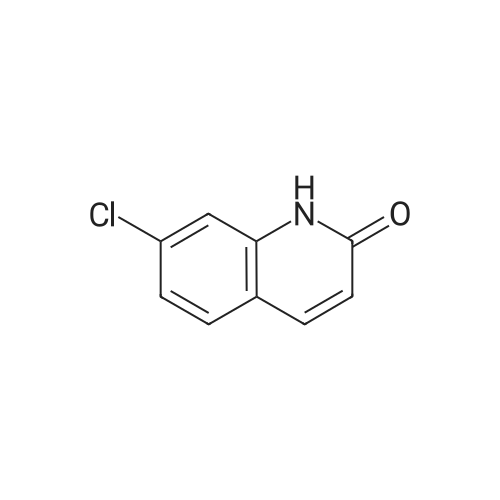
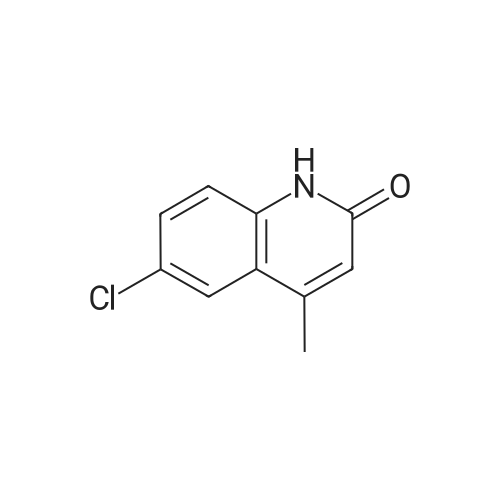
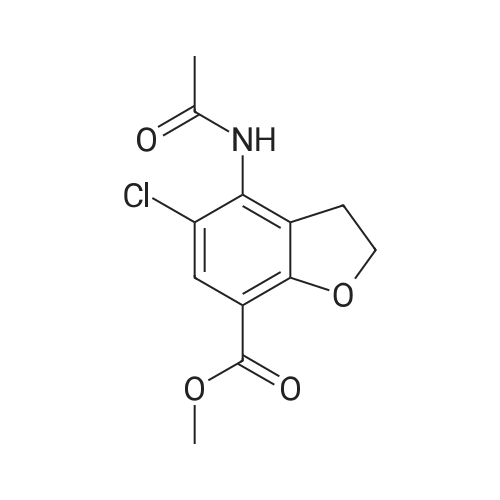
|
With pyridine; In tetrahydrofuran; at 20℃; |
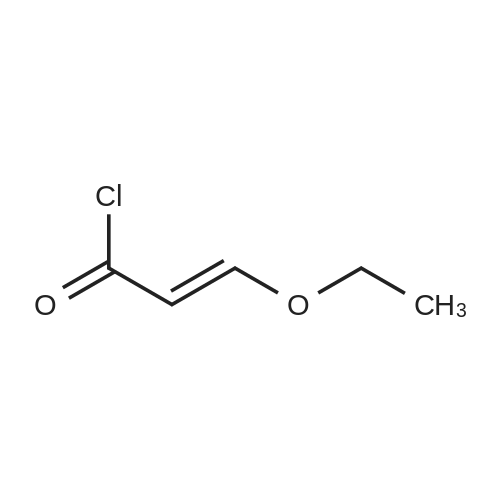
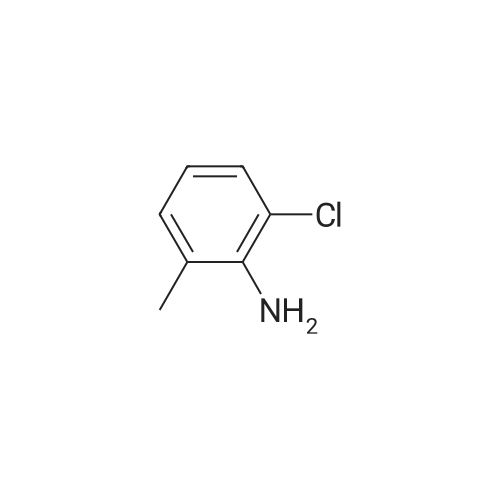
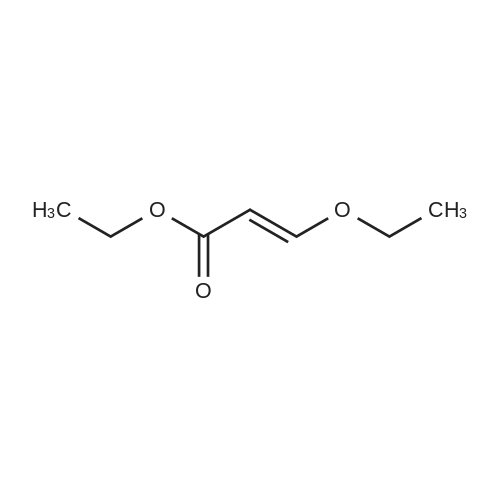
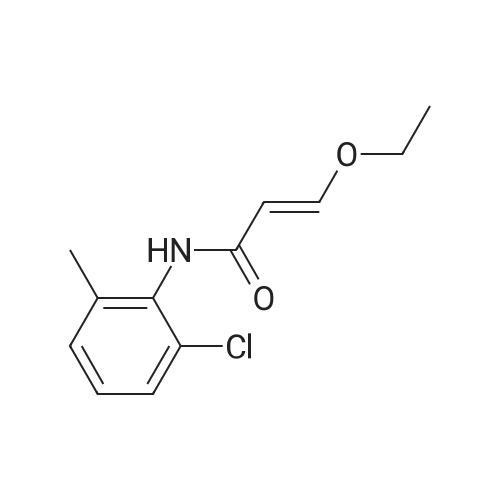
Example 1; [0163] A mixture of ethyl beta-ethoxyacrylate (26.50 g, 183 rnmol) and 2 N sodium hydroxide(110 mL, 220 mrnol) was refluxed for 2 h and cooled to 0C , water was removed under vacc, and the yellow solids were triturated with toluene and evaporated to give the sodium beta- ethoxyacrylate (25 g, 97%). The mixture of sodium beta-thoxyacrylate (10.26 g, 74.29 mrnol) and thionyl chloride (25 mL, 343 mmol) was refluxed for 2 h, and evaporated, to give the beta- ethoxyacryloyl chloride crude product, which was used without purification, To a cold stirring solution of 3-ethoxyacryloyl chloride in THF (100 mL) was added 2-chloro-6-methylaniline (6.2 mL, 50,35 mmol) and pyridine (9 ml, 1 1 1 mmol) The mixture was then wanned and stirred overnight at room temperature. Water was added at 0-10C, extracted with EtOAc, The organic layer was washed with CuSO4 (3x50 mL) and the resulting solution was passed a pad of silica gel, concentrated under vacuum to give solids, The solids was diluted with toluene and kept, at 0C, The solid was collected by vacuum filtration, washed with water and dried to give 5,2 g (43% yield) of compound 1 , (E)-N-(2-chloro-6-methylphenyl)-3-ethoxyacrylamide). 1H NMR (500 Hz1 CDCl3) delta 1 ,26 (t, 3H1 J=7 Hz), 2, 15 (s, 3H), 3 94 (q, 2H, J=7 Hz), 5,58 (d, IH, J=12.4 Hz), 7.10-7.27 (m, 2H, J=7.5 Hz), 7,27-7.37 (d, IH, J=7.5 Hz), 7,45(d, 1Hf J=12.4 Hz); ESI-MS: calcd for (Cl 2H14C1NO2) 239, found 240 MH+), |
|
With pyridine; In tetrahydrofuran; at 20℃; |
Example 1[0156] A mixture of ethyl beta-ethoxyacrylate (26.50 g, 183 mmol) and 2 N sodium hydroxide (110 mL, 220 mmol) was refluxed for 2 h and cooled to 0 C water was removed under vacc, and the yellow solids were triturated with toluene and evaporated to give the sodium beta-ethoxyacrylate (25 g, 97%). The mixture of sodium beta-thoxyacrylate (10.26 g, 74.29 mmol) and thionyl chloride (25 mL, 343 mmol) was refluxed for 2 h, and evaporated, to give the beta-ethoxyacryloyl chloride crude product, which was used without purification. To a cold stirring solution of 3-ethoxyacryloyl chloride in THF (100 mL) was added 2-chloro-6- methylaniline (6.2 mL, 50.35 mmol) and pyridine (9 ml, 111 mmol). The mixture was then warmed and stirred overnight at room temperature. Water was added at 0-10 C, extracted with EtOAc. The organic layer was washed with CuSO4 (3x50 mL) and the resulting solution was passed a pad of silica gel, concentrated under vacuum to give solids. The solids was diluted with toluene and kept, at O0C. The solid was collected by vacuum filtration, washed with water and dried to give 5.2 g (43% yield) of compound 1, (E)-N-(2-chloro-6- methylphenyl)-3-ethoxyacrylamide). 1H NMR (500 Hz, CDCl3) delta 1.26 (t, 3H, J=7 Hz), 2.15 (s, 3H), 3.94 (q, 2H, J=7 Hz), 5.58 (d, IH, J=12.4 Hz), 7.10-7.27 (m, 2H, J=7.5 Hz), 7.27- 7.37 (d, IH, J=7.5 Hz), 7.45(d, IH, J=12.4 Hz); ESI-MS: calcd for (C12Hi4ClNO2) 239, found 240 MH+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping