|
With hydrogen;5%-palladium/activated carbon; In methanol; under 1500.15 Torr; for 2h; |
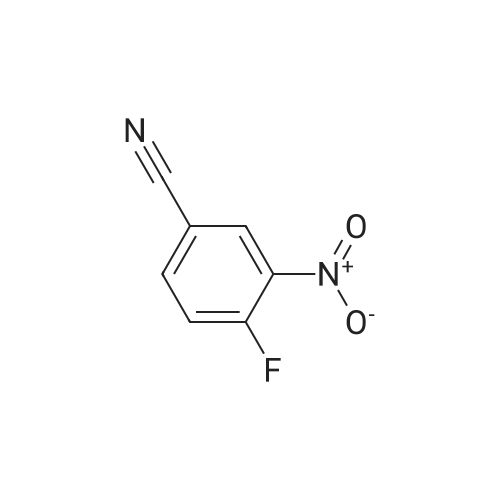
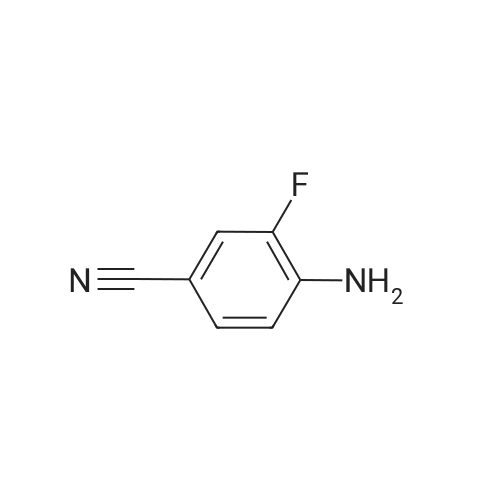
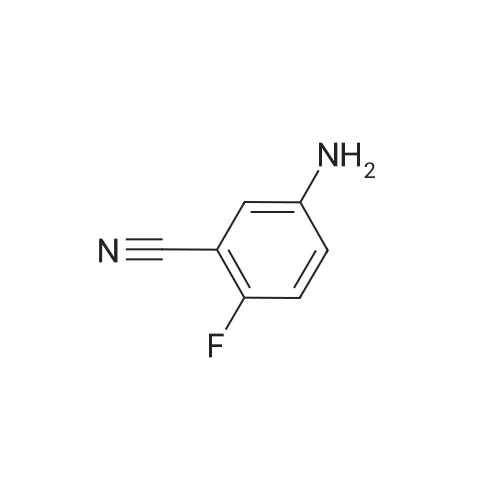
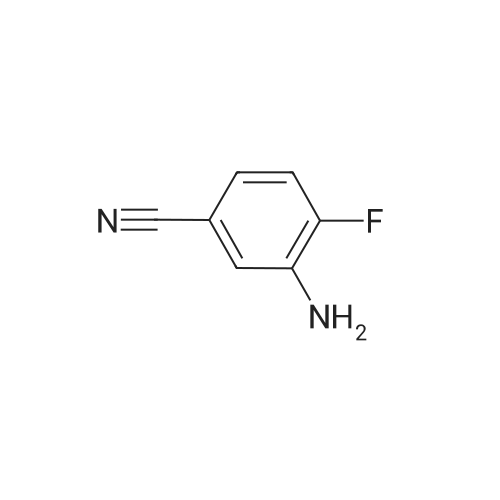
4-fluoro-3-nitrobenzonitrile (5 g, 30 mmol) is first of all subjected to catalytic hydrogenation with 5% palladium on activated charcoal (0.4 g) in 300 mL methanol (2 h at 2 bar hydrogen pressure), whereupon the 3-amino-4-fluorobenzonitrile formed is obtained as a solid after filtration and evaporation of the solvent. Yield: 4.1 g. |
|
With iron; acetic acid; at 80℃; for 1h;Inert atmosphere; |
A mixture of 4-fluoro-3-nitrobenzonitrile (5.0 g, 30.1 mmol) and Fe powder (5.05 g, 90.3 mmol) in AcOH (100 mL) was heated at 80 C. for 1 hour under N2. Then the solvent was removed under vacuum and water (200 mL) was added to the residue. The solution was adjusted to pH 6 by addition of Na2CO3 and extracted with DCM (2×200 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated to yield 3-amino-4-fluorobenzonitrile (48), which was used without further purification. MS m/z 137.0 (M+1)+.; To a stirring suspension of imidazo[1,2-a]pyridine-3-carboxylic acid (1) (3.0 g, 18.5 mmol) in anhydrous dichloromethane (50 mL) at 0 C. was added dropwise oxalyl chloride (4.84 mL, 55.5 mmol). Then, three drops of anhydrous DMF was added and the reaction mixture was stirred at room temperature for 15 minutes. The solvent was concentrated and the crude solid was added to a stirring solution of 3-amino-4-fluorobenzonitrile (48) (2.5 g, 18.5 mmol) in anhydrous pyridine (50 mL) at room temperature. The reaction was stirred for 20 minutes and quenched with water (200 mL) with stirring for another 10 minutes. Then the precipitate was filtered and dried in air to yield N-(5-cyano-2-fluorophenyl)imidazo[1,2-a]pyridine-3-carboxamide (49). 1H NMR (400 MHz, d6-DMSO) delta 10.40 (s, 1H), 9.43 (td, J=1.2, 6.8 Hz, 1H), 8.63 (s, 1H), 8.21 (dd, J=2.0, 7.2 Hz, 1H), 7.78-7.84 (m, 2H), 7.54-7.63 (m, 2H), 7.22 (dt, J=1.2, 6.8, 1H). MS m/z 281.1 (M+1)+.; NH2OH (10 mL, 32.1 mmol) was added in one portion to a stirred suspension of N-(5-cyano-2-fluorophenyl)imidazo[1,2-a]pyridine-3-carboxamide (49) (3.6 g, 12.85 mmol) in EtOH (100 mL). The resulting suspension was heated at 70 C. for 3 hours and then the solvent was removed to yield N-(2-fluoro-5-(N?-hydroxycarbamimidoyl)phenyl)imidazo[1,2-a]pyridine-3-carboxamide (50). 1H NMR (400 MHz, d6-DMSO) delta 10.21 (s, 1H), 9.70 (s, 1H), 9.45 (td, J=1.2, 7.2 Hz, 1H), 8.61 (s, 1H), 7.95 (dd, J=2.4, 7.6 Hz, 1H), 7.79 (td, J=1.2, 8.8 Hz, 1H), 7.51-7.60 (m, 2H), 7.31-7.37 (m, 1H), 7.19 (dt, J=1.2, 6.8, 1H), 5.88 (s, 2H). MS m/z 314.1 (M+1)+. |
|
|
A mixture of 4-fluoro-3-nitrobenzonitrile (5.0 g, 30.1 mmol) and Fe powder (5.05 g, 90.3 mmol) in AcOH (100 mL) was heated at 80 C for 1 hour under N2. Then the solvent was removed under vacuum and water (200 mL) was added to the residue. The solution was adjusted to pH 6 by addition of Na2C03 and extracted with DCM (2 x 200 mL). The organic layers were combined, dried over Na2S04, filtered and concentrated to yield 3- amino-4-fluorobenzonitrile (48), which was used without further purification. MS m/z 137.0 (M+1 ) +. |
|
|
A mixture of 4-fluoro-3-nitrobenzonitrile (5.0 g, 30.1 mmol) and Fe powder (5.05 g, 90.3 mmol) in AcOH (100 mL) was heated at 80 C. for 1 hour under N2. Then the solvent was removed under vacuum and water (200 mL) was added to the residue. The solution was adjusted to pH 6 by addition of Na2CO3 and extracted with DCM (2×200 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated to yield 3-amino-4-fluorobenzonitrile (48), which was used without further purification. MS m/z 137.0 (M+1)+. |
|
With iron; acetic acid; at 80℃; for 1h;Inert atmosphere; |
A mixture of 4-fluoro-3-nitrobenzonitrile (5.0 g, 30.1 mmol) and Fe powder (5.05 g, 90.3 mmol) in AcOH (100 mL) was heated at 80 C for 1 hour under N2. Then the solvent was removed under vacuum and water (200 mL) was added to the residue. The solution was adjusted to pH 6 by addition of Na2C03 and extracted with DCM (2 x 200 mL). The organic layers were combined, dried over Na2S04, filtered and concentrated to yield 3- amino-4-fluorobenzonitrile (48), which was used without further purification. MS m/z 137.0 (M+1 ) +. To a stirring suspension of imidazo[1 ,2-a]pyridine-3-carboxylic acid (1 ) (3.0 g, 18.5 mmol) in anhydrous dichloromethane (50 mL) at 0 C was added dropwise oxalyl chloride (4.84 mL, 55.5 mmol). Then, three drops of anhydrous DMF was added and the reaction mixture was stirred at room temperature for 15 minutes. The solvent was concentrated and the crude solid was added to a stirring solution of 3-amino-4- fluorobenzonitrile (48) (2.5 g, 18.5 mmol) in anhydrous pyridine (50 mL) at room temperature. The reaction was stirred for 20 minutes and quenched with water (200 mL) with stirring for another 10 minutes. Then the precipitate was filtered and dried in air to yield N-(5-cyano-2-fluorophenyl)imidazo[1 ,2-a]pyridine-3-carboxamide (49). 1 H NMR (400MHz, c/6-DMSO) delta 10.40 (s, 1 H), 9.43 (td, J = 1 .2, 6.8 Hz, 1 H), 8.63 (s, 1 H), 8.21(dd, J = 2.0, 7.2 Hz, 1 H), 7.78-7.84 (m , 2H), 7.54-7.63 (m , 2H), 7.22 (dt, J = 1 .2, 6.8, 1 H). MS m/z 281 .1 (M+1 ) +. NH2OH (10 mL, 32.1 mmol) was added in one portion to a stirred suspension of N-(5- cyano-2-fluorophenyl)imidazo[1 ,2-a]pyridine-3-carboxamide (49) (3.6 g, 12.85 mmol) in EtOH (100 mL). The resulting suspension was heated at 70 C for 3 hours and then the solvent was removed to yield N-(2-fluoro-5-(N'- hydroxycarbamimidoyl)phenyl)imidazo[1 ,2-a]pyridine-3-carboxamide (50). 1 H NMR (400MHz, c/6-DMSO) delta 10.21 (s, 1 H), 9.70 (s, 1 H), 9.45 (td, J = 1 .2, 7.2 Hz, 1 H), 8.61 (s, 1 H), 7.95 (dd, J = 2.4, 7.6 Hz, 1 H), 7.79 (td, J = 1 .2, 8.8 Hz, 1 H), 7.51 -7.60 (m , 2H), 7.31 -7.37 (m, 1 H), 7.19 (dt, J = 1 .2, 6.8, 1 H), 5.88 (s, 2H). MS m/z 314.1 (M+1 ) +. |
|
With sodium hydrogencarbonate; tin(ll) chloride; In ethyl acetate; |
4-Fluoro-3-nitrobenzonitrile (1) (2 g, 0.012 mol) in ethyl acetate(50 mL) was supplemented with stannous chloride (11.4 g,0.06 mol). The reaction mixture was stirred for 30 min and then a saturated solution of sodium hydrogenocarbonate (100 mL) was added. This mixture was treated with ethyl acetate (300 mL) andthe aqueous layer was separated. The ethyl acetate layer was evaporated under reduced pressure. The crude product was dissolved in methanol, and ice was added; the resulting precipitate was collected by filtration (Yield: 30e60%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping