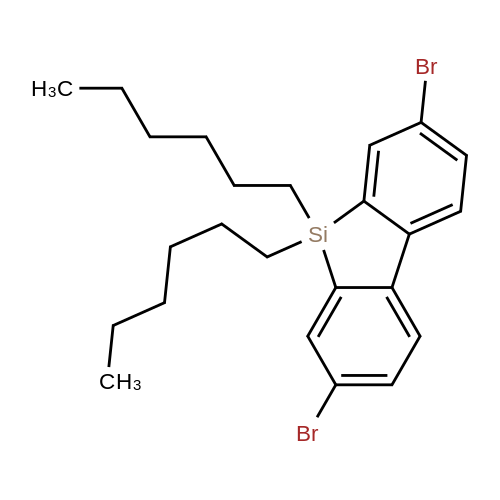
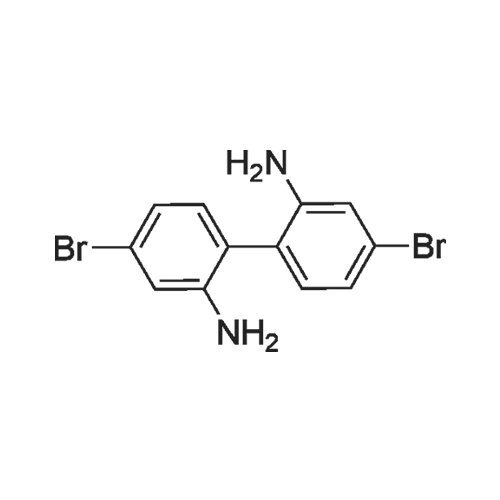
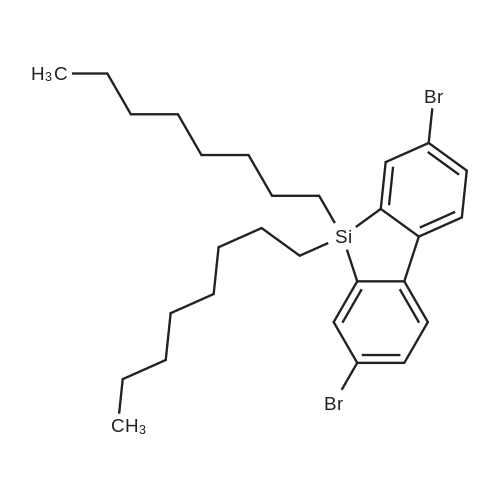
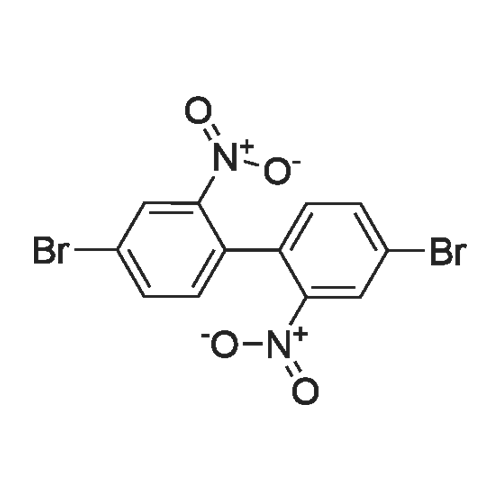
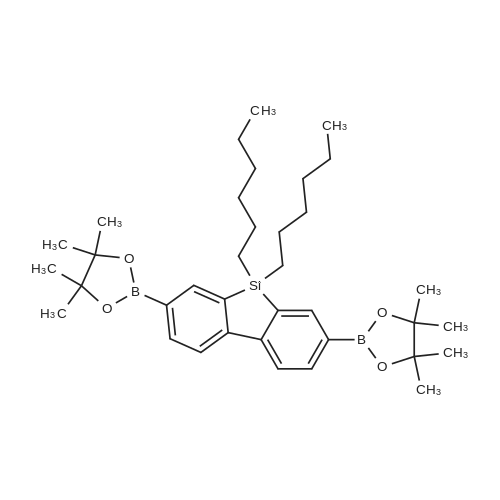
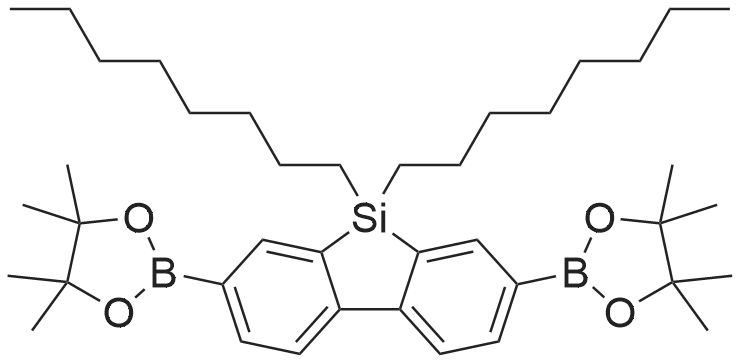
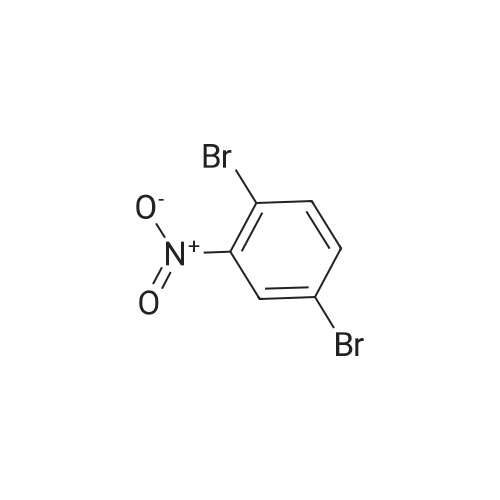
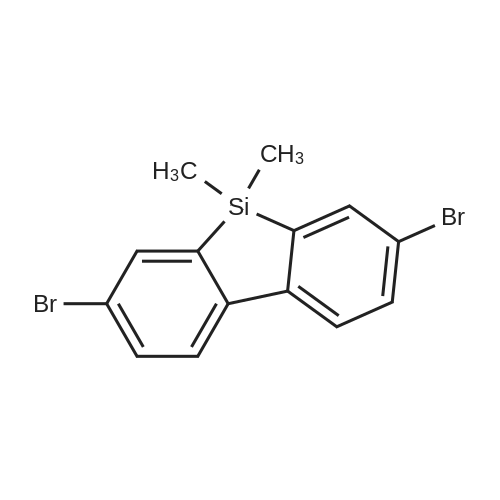
| 29.1% |
|
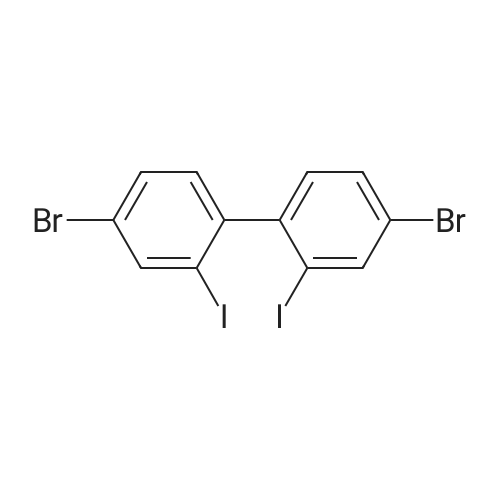
87.7 g (0.256 mol) of 4,4'-dibromo-2,2'-diaminobiphenyl,380 mL of 12 M HCl, and 380 mL of water.The temperature of the reactor was lowered to 0 C.Sodium nitrite (44.2 g, 0.641 mol) was dissolved in 220 mL of water, slowly added dropwise to the reactor,When the addition was complete, the mixture was stirred at the same temperature for 1 hour for 1 hour.Potassium iodide was dissolved in 850 mL of water and slowly added dropwise. When the dropwise addition was completed, the solution was stirred at room temperature for 1 hour.The temperature of the reactor was raised to 60 DEG C and stirred for 3 hours.When the reaction was complete, the mixture was cooled to room temperature and extracted with Ethylacetate to separate the organic layer.The separated organic layer was dried over anhydrous, dried and then subjected to column chromatography using hexane as a developing solvent to obtain 42.1 g (yield: 29.1%) of 4,4'-dibromo-2,2'-diiodobiphenyl. |
| 29.1% |
|
87.7 g (0.256 mol) of 4,4'-Dibromo-2,2'-diaminobiphenyl, 380 mL of 12 M HCl, and 380 mL of water were added thereto. The temperature of the reactor was lowered to 0 C.Sodiumnitrite 44.2 g (0.641 mol) was dissolved in 220 mL of water and slowly added dropwise to the reactor, and when the dropwise addition was completed, the mixture was stirred for 1 hour at the same temperature for 1 hour.Potassium iodide was dissolved in 850 mL of water and slowly added dropwise, and when the addition was completed, the mixture was stirred at room temperature for 1 hour.And the temperature of the reactor was heated up to 60 degreeC and stirred for 3 hours. After the reaction was completed, the reaction mixture was cooled to room temperature and extracted with ethylacetate to separate the organic layer. The organic layer was dried over anhydrous and dried, and then separated by column chromatography using hexane as a developing solvent, to obtain 42.1g (29.1%) of 4,4'-dibromo-2,2'-diodiobiphenyl. |
| 29.1% |
|
87.7 g (0.256 mol) of 4,4'-Dibromo-2,2'-diaminobiphenyl, 380 mL of 12 M HCl, and 380 mL of water were added thereto. The temperature of the reactor was lowered to 0 C.Dissolve 44.2 g (0.641 mol) of sodium nitrite in 220 mL of water and slowly add it to the reactor,When the dropwise addition was completed, the mixture was stirred for 1 hour at the same temperature.Potassium iodide was dissolved in 850 mL of water and slowly added dropwise, and when the addition was completed, the mixture was stirred at room temperature for 1 hour. And the temperature of the reactor was heated up to 60 degreeC and stirred for 3 hours.After the reaction was completed, the reaction mixture was cooled to room temperature and extracted with Ethylacetate to separate the organic layer.The organic layer was dried over anhydrous and dried, and then separated by column chromatography using hexane as a developing solvent, to obtain 42.1g (29.1%) of 4,4'-dibromo-2,2'-diodiobiphenyl. |
| 29% |
|
[Intermediate 5-b] (87.7 g, 0.256 mol) synthesized in [Scheme 5-2] was added to 380 mL of 12 M hydrochloric acid and 380 mL of distilled water.Put the sodium nitrite (44.2 g, 0.641 mol) at 0 was dissolved in 220 mL of distilled water, slowly added dropwise and stirred for 1 hour. Potassium iodide was dissolved in 850 mL of distilled water, slowly added dropwise, and stirred at room temperature for 1 hour. The temperature was raised to 60 C. and stirred for 3 hours. Extracted with ethyl acetate and separated by column chromatography to obtain [Intermediate 5-c] 42.1 g (yield 29%). |
| 29.1% |
|
4,4'-dibromo-2,2'-diaminobiphenyl87.7 g (0.256 mol), 380 mL 12 M HCl,380 mL of water was added. The temperature of the reactor was lowered to 0 C.44.2 g (0.641 mol) of sodiumnitrite was dissolved in 220 mL of water and slowly added dropwise to the reactor,When the dropwise addition was completed, the mixture was stirred for 1 hour at the same temperature for 1 hour. Potassium iodide was dissolved in 850 mL of water and slowly added dropwise.When the dropwise addition was completed, the mixture was stirred at room temperature for 1 hour. And the temperature of the reactor was raised to 60 C. and stirred for 3 hours.When the reaction was completed, the mixture was lowered to room temperature and extracted using Ethylacetate to separate the organic layer.The separated organic layer was dried by anhydrous treatment, and then separated by column chromatography using hexane as a developing solvent.42.1 g (29.1%) of 4,4'-dibromo-2,2'-diiodobiphenyl was obtained. |
| 27% |
|
Step ^ - Preparation of Compound Int-43bTo a stirred suspension of 2,2'-diamino-4,4'-dibromobiphenyl Int-43a (11.2 g, 33 mmol) (prepared from J. Am. Chem. Soc. 2005, 127, 7662) in cone. HC1 (50 mL)/water (50 mL) at 0 C was added a 50% soln of NaN02 in water (30 mL, 73 mmol) over 30 minutes while maintaining the temperature <5 C. The mixture was allowed to stir for an additional 30 minutes whereupon a soln of KI (54 g, 0.33 mol) in water (120 mL) was added dropwise over 1 h. The resulting mixture was heated to 60 C and was allowed to stir for 5h. The mixture was cooled to room temperature and was filtered. The resultant solid was washed with EtOAc (~ 500 mL) and the resultant filtrate was washed with brine (2 x 50 mL), dried (Na2S04), filtered, and concentrated in vacuo. The crude product was purified using flash chromatography using 100% hexanes to provide 5.1 g (27%) of compound Int-43b as a colorless oil. |
| 25% |
With toluene-4-sulfonic acid; potassium iodide; sodium nitrite; In water; acetonitrile; at 20℃;Inert atmosphere; |
(3) The compound 3 (7.16 g, 20 mmol) and p-toluenesulfonic acid (17.18 g, 90.4 mmol) were dissolved in 200 mL of acetonitrile, stirred at 0 C for half an hour, and sodium nitrite (4.14, 1) and potassium iodide (12.45 g, 75 mmol) were dissolved in 40 mL of water and added dropwise to the reaction flask with a constant pressure dropping funnel, which was then allowed to warm to room temperature and allowed to react overnight. After completion of the reaction, the saturated sodium thiosulfate solution was added to the reaction solution and the resulting iodine was extracted and extracted with methylene chloride. The organic phase was collected and dried over anhydrous sodium sulfate. The product was purified by silica gel chromatography using petroleum ether as eluent The residue was purified by column chromatography and dried in vacuo to give a white solid 4 in a yield of 25% |
| 23% |
|
4,4'-Dibromo-1,1'-biphenyl-2,2'-diamine(16g, 46.8 mmol) dissolved in a mixture of 56 mL hydrochloric acid and 64 mL water.Stir under ice bath. after that,An aqueous solution of 40 mL of sodium nitrite (8 g, 116 mmol) was slowly added dropwise to the reaction solution, and the temperature was constant at about 0 C during the dropwise addition. After the addition is completed,The mixture was stirred for 30 minutes, and an aqueous solution of potassium iodide (77 g dissolved in 150 mL of water) was added dropwise to the reaction solution at -5 C.After the reaction at room temperature for 1 hour, the temperature was raised to 60 C for 3 hours.The solution turned dark brown. After the reaction is completed, the reaction solution is suction filtered.Purified by column chromatography (silica gel, n-hexane) to give a white solid(6.07g, 23%). |
| 18% |
|
To a mixture of 4,4'-Dibromobiphenyl-2,2'-diamine (1.8 g, 4.5 mmol) and 8 N HCl aqueous solution (35 mL), an aqueous solution of sodium nitrite (0.7 g, 10.1 mmol) was added dropwise at 0 C. After stirring for 30 min, a solution of KI (1.6 g, 9.6 mmol) in water (5 ml) was slowly added during 5 min. Then, the mixture was warmed up to room temperature for 1 h. The mixture was extracted with CH2Cl2 (3×30 mL). The organic phase was washed with saturated sodium thiosulfate solutionan and water, and dried over anhydrous Na2SO4. After concentrated under vacuum, the crude product was purified by column chromatography silica gel with petroleum ether to get white solid (0.5 g, 18%). 1H NMR (400 MHz, CDCl3): 8.09 (d, J = 2.0 Hz, 2H), 7.55 (dd, J = 8.4, 2.0 Hz, 2H), 7.03 (d, J = 8.0 Hz, 2H). |
|
|
To 36% hydrochloric acid/water (75 ml/85 ml) was added 21.3 g of the compound (10-c), and to this, an aqueous NaNO2 solution (NaNO2 10.7 g/water 55 ml) was added dropwise at 5C. The resulting mixture was stirred at 5C for 30 minutes, and then an aqueous KI solution (KI 104 g/water 200 ml) was added dropwise and the resulting mixture was stirred at 5C for 1 hour, at room temperature for 1 hour and at 60C for 3 hours. A solid fraction produced was collected by filtration and purified by column chromatography (filler: silica gel, eluent: hexane) to obtain 4.27 g of compound (10-d). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping