| 97% |
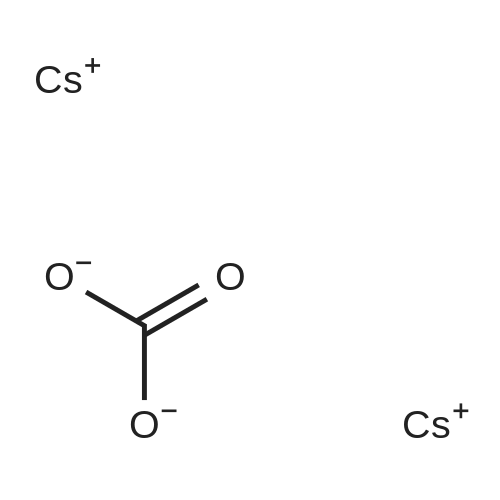
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; |
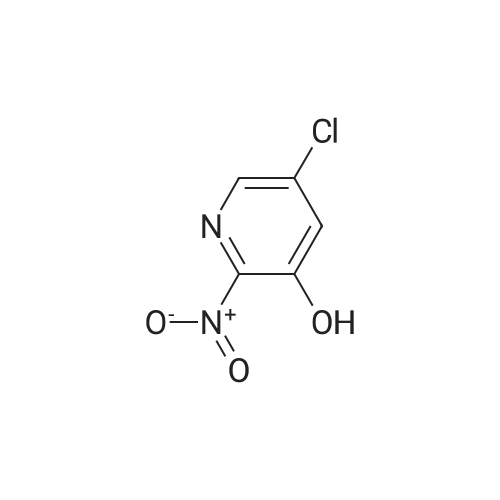
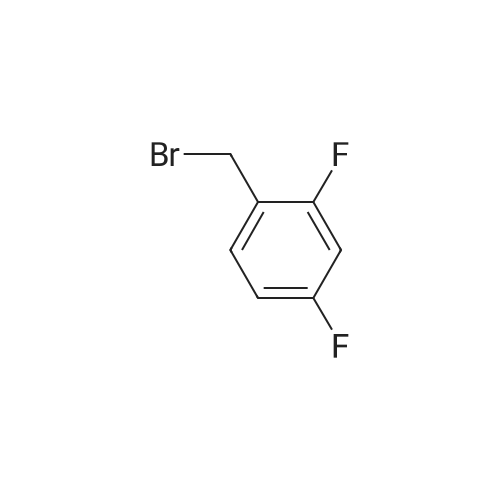
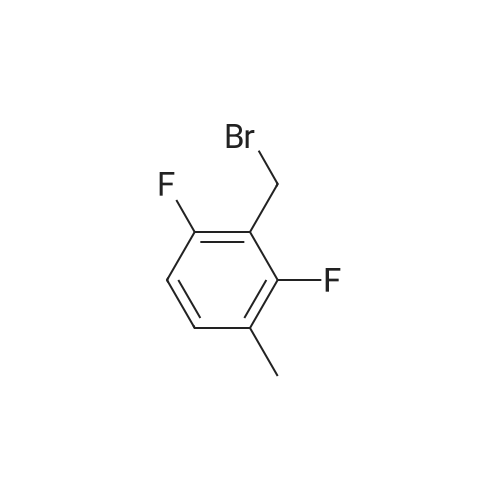
33 g of <strong>[936247-35-7]5-chloro-2-nitropyridin-3-ol</strong> (Example 7A; 189 mmol, 1 equivalent) and 61.6 g of caesium carbonate (189 mmol, 1 equivalent) were initially charged in 528 ml of DMF, 40.4 g of 2,6-difluorobenzyl bromide (189 mmol, 1 equivalent) were added and the mixture was stirred at RT overnight. The reaction mixture was stirred into water/1N aqueous hydrochloric acid. The solid was filtered off, washed with water and air-dried. This gave 54.9 g (97% of theory) of the title compound. 1H-NMR (400 MHz, DMSO-d6): delta=5.46 (s, 2H), 7.22 (t, 2H), 7.58 (q, 1H), 8.28 (d, 1H), 8.47 (d, 1H). |
| 97% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; |
Example 8A 5-Chloro-3-[(2,6-difluorobenzyl)oxy]-2-nitropyridine 33 g of <strong>[936247-35-7]5-chloro-2-nitropyridin-3-ol</strong> (Example 7A; 189 mmol, 1 equivalent) and 61.6 g of caesium carbonate (189 mmol, 1 equivalent) were initially charged in 528 ml of DMF, 40.4 g of 2,6-difluorobenzyl bromide (189 mmol, 1 equivalent) were added and the mixture was stirred at RT overnight. The reaction mixture was stirred into water/1 N aqueous hydrochloric acid. The solid was filtered off, washed with water and air-dried. This gave 54.9 g (97% of theory) of the title compound. 1H-NMR (400 MHz, DMSO-d6): delta=5.46 (s, 2H); 7.22 (t, 2H); 7.58 (q, 1H); 8.28 (d, 1H); 8.47 (d, 1H). |
| 97% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; |
33 g of <strong>[936247-35-7]5-chloro-2-nitropyridin-3-ol</strong> (Example 4A;189 mmol, 1 equivalent) and 61.6 g of caesium carbonate(189 mmol, 1 equivalent) were initially charged in 528 ml ofDMF, 40.4 g of 2,6-difluorobenzyl bromide (189 mmol, 1equivalent) were added and the mixture was stirred at RTovernight. The reaction mixture was stirred into water/iNaqueous hydrochloric acid. The solid was filtered off,washed with water and air-dried. This gave 54.9 g (97% oftheory) of the title compound. ?H-NMR (400 MHz, DMSO-d5): oe=5.46 (s, 2H);7.22 (t, 2H); 7.58 (q, 1H); 8.28 (d, 1H); 8.47 (d, 1H). |
| 97% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; |
33 g of <strong>[936247-35-7]5-chloro-2-nitropyridin-3-ol</strong> (Example 1A; 189 mmol, 1 equivalent) and 61.6 g of caesium carbonate (189 mmol, 1 equivalent) were initially charged in 528 ml of DMF, 40.4 g of 2,6-difluorobenzyl bromide (189 mmol, 1 equivalent) were added and the mixture was stirred at RT overnight. The reaction mixture was stirred into water/1N aqueous hydrochloric acid. The solid was filtered off, washed with water and air-dried. This gave 54.9 g (97% of theory) of the title compound. 1H-NMR (400 MHz, DMSO-d6): delta=5.46 (s, 2H); 7.22 (t, 2H); 7.58 (q, 1H); 8.28 (d, 1H); 8.47 (d, 1H). |
| 97% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; |
33 g of <strong>[936247-35-7]5-chloro-2-nitropyridin-3-ol</strong> (Example12A; 189 mmol, 1 equivalent) and 61.6 g of caesium carbonate (189 mmol, 1 equivalent) were initially charged in 528 ml of DMF, 40.4 g of 2,6-difluorobenzyl bromide (189 mmol, 1 equivalent) were added and the mixture was stirred at RT overnight. The reaction mixture was stirred into a mixture of water/iN hydrochloric acid, and the crystals were filtered off with suction, washed with water and air-dried. 54.9 g (97% of theory) of the title compound were obtained. j0377] ?H-NMR (400 MHz, DMSO-d5): oe=5.46 (s, 2H); 7.22 (t, 2H); 7.58 (quint., 1H); 8.28 (d, 1H); 8.47 (d, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping