|
With 1-methyl-pyrrolidin-2-one; sodium hydroxide; In tetrahydrofuran; |
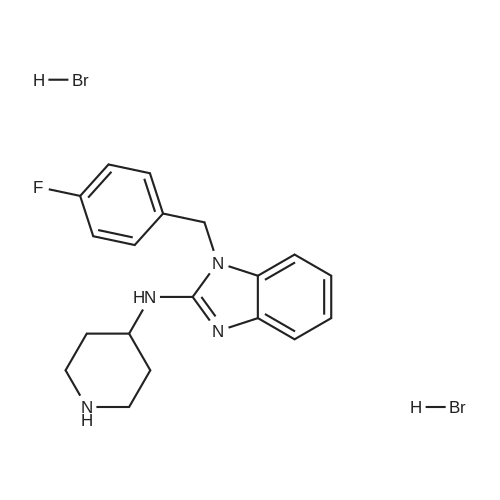
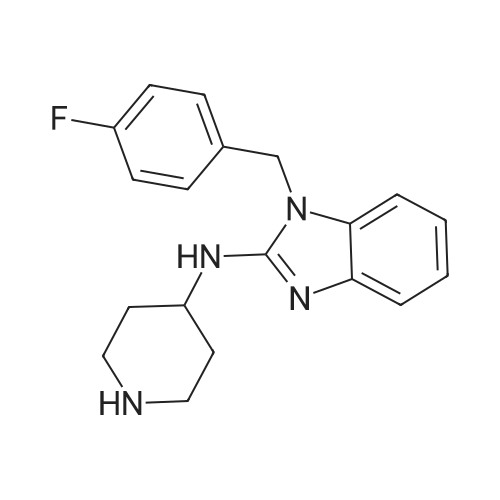
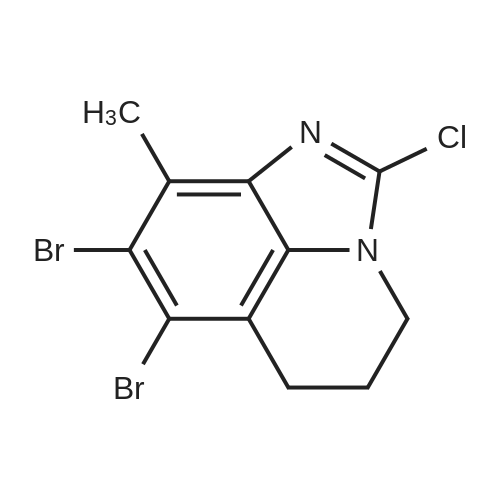
EXAMPLE 42 To a 25 mL 3-neck flask equipped with a thermometer, reflux consenser and stir bar were added, under a nitrogen atmosphere, 885.6 mg (4.8 mmol) of Compound XXII, obtained according to the procedure of Example 41, and 0.93 mL (8.0 mmol) of lutidine. The mixture was heated to 120 C., whereupon a solution of 1.04 g (4 mmol., 1 eq.) of Compound X (Aldrich Chemical Co., Milwaukee, Wis.), 8 mmol of tetrabutylammonium fluoride (obtained from its 1.0M THF solution by distillation under vacuum with anhydrous toluene) and 4 mL of N-methylpyrrolidinone was added slowly over 2 h. The reaction mixture was allowed to stir at 120 C. for 1 h. High-performance liquid chromatography revealed >95% coversion to Compound XXIII. The reaction mixture was allowed to cool to ambient temperature, and slowly poured into a solution of 5% aqueous NaOH while stirring. The resulting suspension was allowed to stir at 0-10 C. for 30 minutes, and the resulting solid product was collected by vacuum filtration, washed with water (3*5 mL), and air dried for 30 minutes under vacuum to afford crude Compound XXIII: 1 H NMR (300 MHz, CDCl3) δ7.52 (1H, d, J=7.8 Hz), 7.20-7.00 (7H, m, two groups), 5.13 (2H, s), 4.40 (1H, NH br. d), 4.32 (2H, pseudo d), 4.20 (1H, m), 3.00 (2H, pseudo t), 2.15 (2H, pseudo d), 1.30 (2H, m overlapped), 1.26 (9H, s); 13 C NMR (75 MHz, CDCl3) δ176.3, 164.2 and 161.0 (13 C-19 F coupling), 153.3, 142.4, 134.6, 131.4, 128.4, 121.7, 120.0, 116.6, 116.4, 116.1, 107.5, 50.5, 45.1, 44.2, 38.9, 33.1, 28.6. It is to be noted that under the same reaction conditions as above, but without the use of tetrabutylammonium fluoride, only 3.5% conversion (high-performance liquid chromatography) to Compound XXIII was achieved after 3 h at 120 C. The Compound XXIII obtained above was placed in a 50 mL, 3-neck flask equipped with a thermometer, reflux condenser and stir bar. 5 mL of 48% hydrobromic acid was added, and the resulting mixture was heated to 110 C. and allowed to stir at that temperature for 2 h. After the reaction was complete (>98% conversion to norastemizole as shown by high-performance liquid chromatography), the reaction mixture was allowed to cool to room temperature, and 10 mL of toluene and 10 mL of water were added, with stirring. |
|
With 1-methyl-pyrrolidin-2-one; sodium hydroxide; In tetrahydrofuran; |
EXAMPLE 43 Norastemizole. To a 25 mL 3-neck flask equipped with a thermometer, ref lux consenser and stir bar were added, under a nitrogen atmosphere, 822 mg (4.8 mmol) of Compound XVI, obtained according to the procedure of Example 6, and 0.93 mL (8.0 mmol) of lutidine. The mixture was heated to 120 C., whereupon a solution of 1.04 g (4 mmol., 1 eq.) of Compound X (Aldrich Chemical Co., Milwaukee, Wis.), 8 mmol of tetrabutylammonium fluoride (obtained from its 1.0M THF solution by distillation under vacuum with anhydrous toluene) and 4 mL of N-methylpyrrolidinone was added slowly over 2 h. The reaction mixture was allowed to stir at 120 C. for 2 h. High-performance liquid chromatography revealed >95% coversion to Compound XVIII. The reaction mixture was allowed to cool to ambient temperature, and slowly poured into a solution of 5% aqueous NaOH while stirring. The resulting suspension was allowed to stir at 0-10 C. for 30 minutes, and the resulting solid product was collected by vacuum filtration, washed with water (3*5 mL), and air dried for 30 minutes under vacuum to afford a crude mixture of Compound XVIII and 6% (HPLC) of migration product Compound XIX. NMR data for Compound XVIII: 1 H NMR (300 MHz, DMSO-d6) δ7.30-7.02 (6H, m two groups), 6.95 (1H, pseudo t, J=7.8, 2.3 Hz), 6.85 (1H, pseudo t, J=7.8, 2.3 Hz), 5.35 (2H, s), 4.33 (1H, pseudo d), 4.02 (1H, m), 3.83 (1H, pseudo d), 3.17 (1H, pseudo t), 2.75 (1H, pseudo t), 2.02 (3H, s), 2.0 (2H, m overlapped), 1.45 (2H, m); 13 C NMR (75 MHz, DMSO-d6) δ168.0, 163.0 and 159.7 (13 C-19 F coupling), 153.9, 142.8, 134.3, 133.5, 129.1, 129.0, 128.2, 120.5, 118.4, 115.5, 115.2, 107.9, 44.8, 43.7, 32.3, 31.5, 21.4. It is to be noted that under the same reaction conditions as above, but without the use of tetrabutylammonium fluoride, only 4.9% conversion (high-performance liquid chromatography) to Compound XVIII was achieved after 3 h at 120 C. In addition, without the use of tetrabutylammonium fluoride, the ratio of Compound XVIII:Compound XIX was 4:1. The mixture of Compound XVIII obtained above was placed in a 50 mL, 3-neck flask equipped with a thermometer, reflux condenser and stir bar. 5 mL of 6N hydrochloric were added, and the resulting mixture was heated to 110 C. and allowed to stir at that temperature for 5 h. After the reaction was complete (>98% conversion to norastemizole as shown by high-performance liquid chromatography), the reaction mixture was allowed to cool to room temperature, and 10 mL of toluene and 10 mL of water were added, with stirring. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping