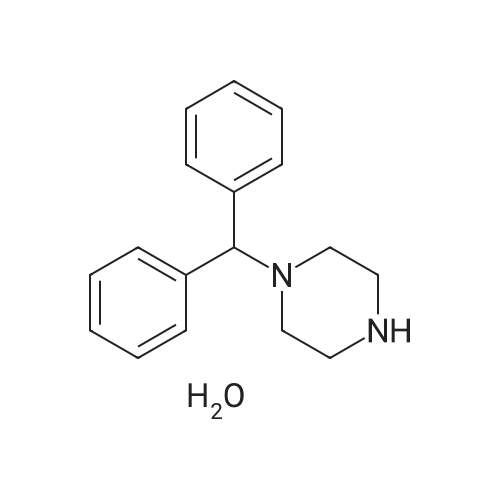
Alternatived Products of [ 841-77-0 ]
Product Details of [ 841-77-0 ]
| CAS No. : | 841-77-0 |
MDL No. : | MFCD00038379 |
| Formula : |
C17H20N2
|
Boiling Point : |
- |
| Linear Structure Formula : | NH(CH2)4NCH(C6H5)2 |
InChI Key : | NWVNXDKZIQLBNM-UHFFFAOYSA-N |
| M.W : |
252.35
|
Pubchem ID : | 70048 |
| Synonyms : |
|
Chemical Name : | 1-Benzhydrylpiperazine |
Safety of [ 841-77-0 ]
| Signal Word: | Warning |
Class: | N/A |
| Precautionary Statements: | P280-P305+P351+P338 |
UN#: | N/A |
| Hazard Statements: | H302 |
Packing Group: | N/A |
| GHS Pictogram: |

|
Application In Synthesis of [ 841-77-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 841-77-0 ]
- 1
-
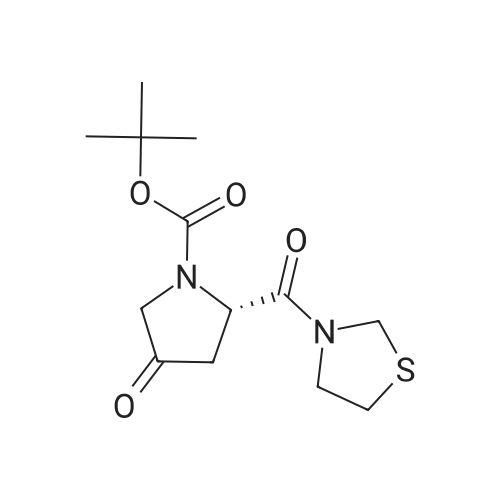 [ 401564-36-1 ]
[ 401564-36-1 ]

-
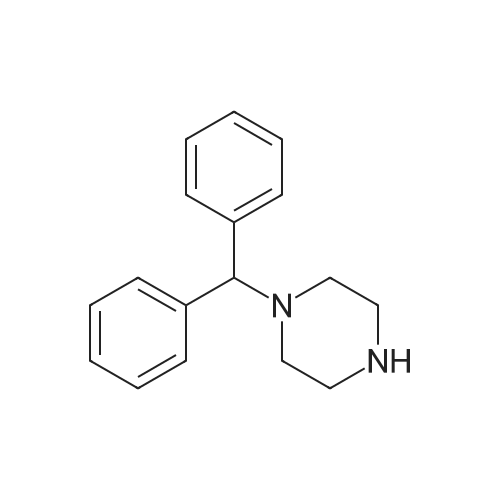 [ 841-77-0 ]
[ 841-77-0 ]

-
 [ 401566-09-4 ]
[ 401566-09-4 ]
- 2
-
 [ 841-77-0 ]
[ 841-77-0 ]

-
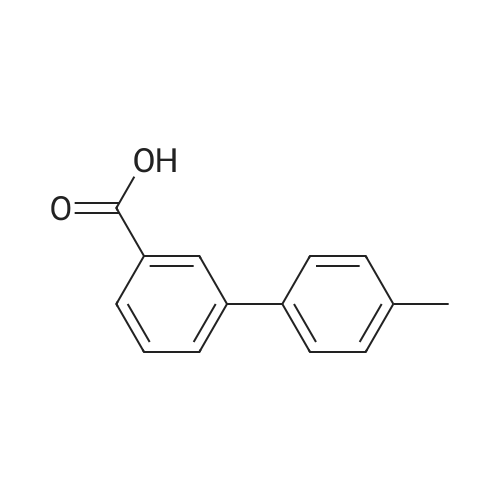 [ 147404-69-1 ]
[ 147404-69-1 ]

-
1-diphenylmethyl-4-(4'-methylbiphenyl-3-carbonyl)piperazine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium hydroxide; thionyl chloride; In N-methyl-acetamide; n-heptane; dichloromethane; |
A. Preparation of (19) where --NR2 R3 represents Diphenylmethylpiperazine, R11 is 4-Methylphenyl, and R12 is Hydrogen To a suspension of <strong>[147404-69-1]4'-methylbiphenyl-3-carboxylic acid</strong> (2.2 g) in a mixture of methylene chloride (20 ml) and dimethylformamide (0.5 ml) was added thionyl chloride (1.48 g). The mixture was refluxed until the suspension dissolved, after which the temperature was allowed to cool to room temperature. To this solution was added 1-(diphenylmethyl)piperazine (3.94 g) in methylene chloride dropwise, and the reaction mixture allowed to stand overnight. Sodium hydroxide (30 ml of 1N) and 50 ml of methylene chloride was added, the organic layer separated, washed with brine, dried over sodium sulfate, and solvent removed under reduced pressure. The residue was chromatographed on silica gel, eluding with 25% ethyl acetate in heptane, to yield 3.8 g of 1-diphenylmethyl-4-(4'-methylbiphenyl-3-carbonyl)piperazine. The dihydrochloride salt was prepared and recrystallized from ethanol, m.p. 226 C. |
- 3
-
 [ 841-77-0 ]
[ 841-77-0 ]

-
 [ 63547-24-0 ]
[ 63547-24-0 ]

-
1-(4-benzhydrylpiperazin-1-yl)-2-(benzhydrylsulfinyl)ethanone
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;dmap; In dichloromethane; at 20℃; |
Example 15; Synthesis of l-(4-benzhydrylpiperazin-l-yl)-2-(4-benzhydrylsulfinyl)ethanone(compound no. 45); [00119] To a solution of 1-diphenylmethylpiperazine 0.125 g (0.5mmol) dissolved in methylene chloride (5 ml) was added 2-(benzhydrylsulfinyl)acetic acid 0.138 g (0.5 mmol) (synthesized according to Example l(e)), EDC 0.191 g (1 mmole) and trace of DMAP, and the reaction mixture was stirred at room temperature overnight. The reaction mixture was concentrated and dissolved in ethyl acetate (10 ml) and washed with saturated sodium bicarbonate solution and brine, dried over sodium sulfate and concentrated. The residue was applied to flash column chromatography using methylene chloride and methanol (100:10) as eluents to give 0.13 g of desired product. |
- 4
-
 [ 841-77-0 ]
[ 841-77-0 ]

-
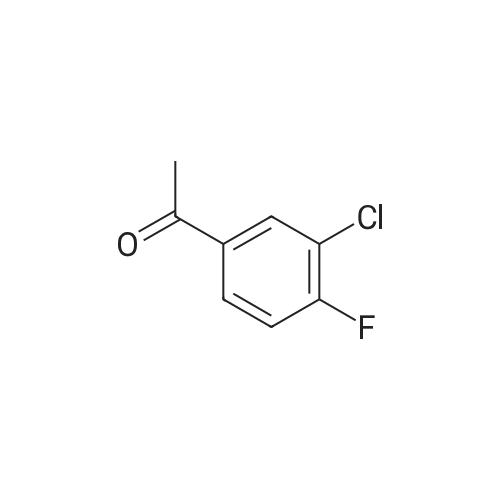 [ 2923-66-2 ]
[ 2923-66-2 ]

-
1-(3-chloro-4-fluorophenyl)-3-[4-(diphenylmethyl)piperazin-1-yl]propan-1-ol
[ No CAS ]
- 5
-
 [ 50-00-0 ]
[ 50-00-0 ]

-
 [ 841-77-0 ]
[ 841-77-0 ]

-
 [ 2923-66-2 ]
[ 2923-66-2 ]

-
C26H26ClFN2O
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In 1,4-dioxane; at 100 - 110℃; under 1034.32 Torr; for 0.0833333h;pH 1 - 2;Microwave irradiation; Sealed tube; |
General procedure: Ketones derivatives were prepared by Lehmann's method [41], which was adapted and optimized. In a 35mL microwave vial, a solution of the corresponding substituted aryl methyl ketone (V) (1.0 equiv), the corresponding aryl amine as a base or hydrochloride salt (IIIa-d, IVa-g) (1.0 equiv), and paraformaldehyde (1.2 equiv) in 1,4-dioxane (4mL) was stirred for 5minat 20-22C. If necessary, concentrated HCl was added dropwise to the mixture until a pH of 1-2 was reached. The resulting mixture was heated using microwave irradiation at 100-110C, 20 psi, and 150W for 5min. Then, the mixture was poured into a flask and the 1,4-dioxane was removed in vacuo. The residue was diluted with H2O (30mL) and basified with 2M NaOH to basic pH and stirred for 1h. The mixture was transferred into a separatory funnel and extracted with DCM (3×50mL). The organic phase was dried with anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure. In some cases, the residue obtained was purified by gradient elution glass-column chromatography on silica gel using DCM/MeOH (v/v) as an eluent or gradient elution automated flash chromatography eluting with DCM/MeOH (v/v), affording the desired aryl-ketone (1-36). Based on our previous SAR studies [13,14], the aryl-ketone derivatives were inactive against the NF54, 3D7, and FCR-3 strains of P.falciparum, and therefore they were not an objective or priority in the project. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping