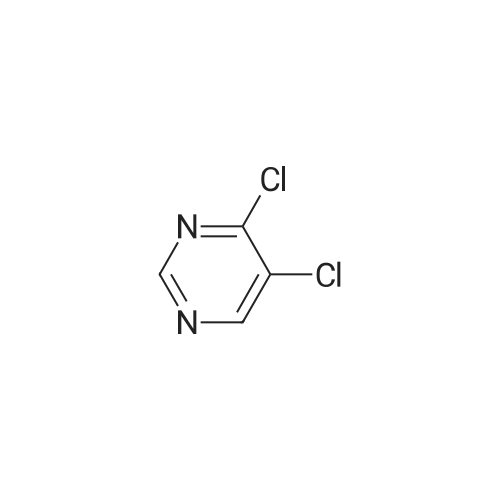
| 88.5% |
With trichlorophosphate; In toluene; at 5 - 7℃;Reflux; |
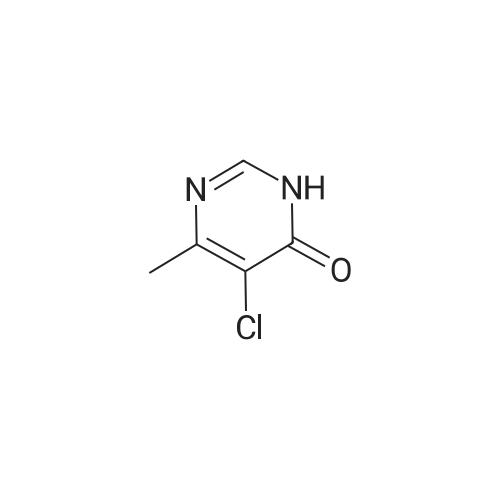
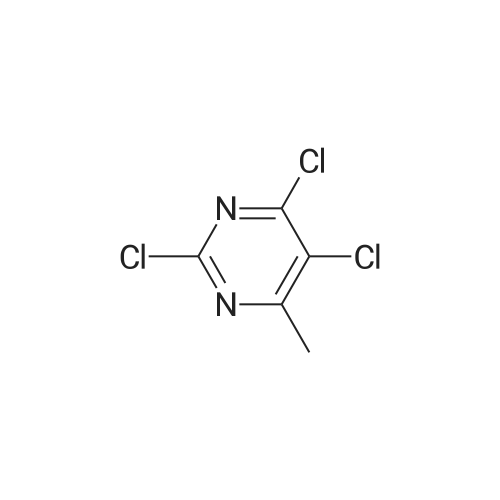
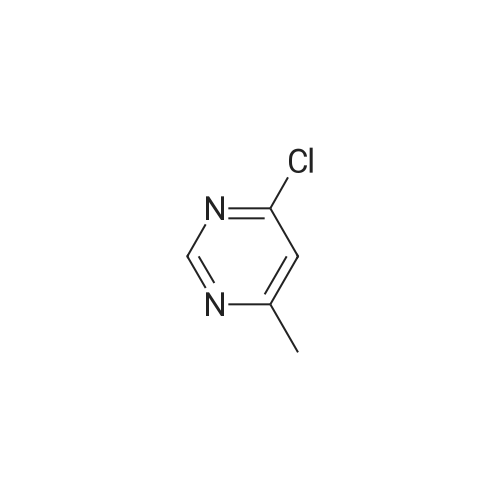
14.5g (0.1mol) 4- hydroxy-5-chloro-6-methylpyrimidine dissolved in 50ml of toluene, was added dropwise with stirring to the reaction flask 50ml phosphorus oxychloride, after dropwise addition the reaction heated at reflux for 5-7 hours, TLC monitored after completion of the reaction. Toluene was evaporated under reduced pressure and excess phosphorus oxychloride, with stirring the reaction was poured into ice water, the aqueous phase was extracted with ethyl acetate (3 × 50ml), the combined organic phase was dried over anhydrous magnesium sulfate, filtered, and dissolved. The residue was purified by column chromatography (eluent, ethyl acetate and petroleum ether (boiling range 60-90 deg. C), the volume ratio of 1: 4) was isolated as a yellow liquid 14.43g, yield 88.5%. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
The 14.5g (0.1mol) 4- hydroxy-5-chloro-6-methylpyrimidine dissolved in 50ml of toluene, was added dropwise with stirring to the reaction flask 50ml of phosphorus oxychloride dropwise at reflux temperature the reaction completion 5-7 hours . After completion of the reaction was monitored by TLC, toluene was distilled off under reduced pressure and an excess of phosphorus oxychloride, while stirring the reaction was poured into ice water, the aqueous phase was extracted with (3 × 50ml) and extracted with ethyl acetate, the combined organic phase was dried over anhydrous magnesium dried, filtered, removing solvent. The residue was purified by column chromatography (eluent, ethyl acetate and petroleum ether, the volume ratio of 1: 5) was isolated yellow liquid 14.43 g, 88.5% yield. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
50 ml of POCl3 was added dropwise to a solution of 14.5 g (0.1 mol) of <strong>[7752-72-9]4-hydroxyl-5-chloro-6-methylpyrimidine</strong> in 50 mL of toluene, the mixture was refluxed for 5-7 h after addition. After the reaction was over by Thin-Layer Chromatography monitoring, the reaction mixture was concentrated under reduced pressure to remove toluene and extra POCl3, and then poured into ice water. The water phase was extracted with ethyl acetate (3*50 mL), the organic phases were emerged, dried over anhydrous magnesium sulfate, filtered and then concentrated under reduced pressure. The residue was purified through silica column (ethyl acetate/petroleum ether=1:5, as an eluent) to give 14.43 g as yellow liquid with yield of 88.5%. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
Dissolve 14.45 g (0.1 mol) of <strong>[7752-72-9]4-hydroxy-5-chloro-6-methylpyrimidine</strong> in 50 ml of toluene and stir it under anti-flask50 ml of phosphorus oxychloride was added dropwise, and the reaction was heated to reflux for 5-7 hours. TLC was monitored after the reaction was completed. Vacuum distillation of toluene andThe amount of phosphorous oxychloride was poured into ice water with stirring, the aqueous phase was extracted with (3×50 ml) ethyl acetate, and the organic phases were combined.Anhydrous magnesium sulfate drying and desolventization. Chromatographic separation of the yellow liquid 14.43g, the yield of 88.5%. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
Dissolve 14.5 g (0.1 mol) of <strong>[7752-72-9]4-hydroxy-5-chloro-6-methylpyrimidine</strong> in 50 ml of toluene, add 50 ml ofphosphorus oxychloride to the reaction flask with stirring, and then heat up to reflux for 5-7 hours. After TLC monitoring was completed, toluene and excess phosphorus oxychloride were distilled off underreduced pressure. The reaction was poured into ice water with stirring. The aqueous phase was extracted with(3×50 ml) ethyl acetate, and the organic phases were combined and dried over anhydrous magnesium sulfate.Dry, filter, and dissolve. The residue was purified by column chromatography (eluent: ethyl acetate and petroleum ether, 1 : 5 in volume) to give 14.43 g of a yellow liquid, yield 88.5%. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
50 ml of POCl3 was added dropwise to a solution of 14.5 g (0.1 mol) of <strong>[7752-72-9]4-hydroxyl-5-chloro-6-methylpyrimidine</strong> in 50 mL of toluene, the mixture was refluxed for 5-7 h after addition. After the reaction was over by Thin-Layer Chromatography monitoring, the reaction mixture was concentrated under reduced pressure to remove toluene and extra POCl3, and then poured into ice water. The water phase was extracted with ethyl acetate (3*50 mL), the organic phases were merged, dried over anhydrous magnesium sulfate, filtered and then concentrated under reduced pressure. The residue was purified through silica column (ethyl acetate/petroleum ether=1:5, as an eluent) to give14.43 g as yellow liquid with yield of 88.5%. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
14.5 g (0.1 mol) of <strong>[7752-72-9]4-hydroxy-5-chloro-6-methylpyrimidine</strong> was dissolved in 50 ml of toluene solution.50 ml of phosphorus oxychloride was added dropwise to the reaction flask under stirring, and the mixture was heated under reflux for 5-7 hours.After the TLC monitoring reaction was completed, toluene and excess phosphorus oxychloride were distilled off under reduced pressure, and the reactant was poured into ice water with stirring.The aqueous phase was extracted with ethyl acetate (3 × 50ml), the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, desolventized.Residue column chromatography (eluent is ethyl acetate and petroleum ether,Volume ratio of 1: 5) to give yellow liquid was isolated 14.43g, yield 88.5%. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
Dissolve 14.5g (0.1mol) <strong>[7752-72-9]4-hydroxy-5-chloro-6-methylpyrimidine</strong> in 50ml toluene solution,Under stirring, 50ml of phosphorus oxychloride was dropped into the reaction bottle, and the temperature was raised and the reaction was refluxed for 5-7 hours.After the reaction was monitored by TLC, toluene and excess phosphorus oxychloride were distilled off under reduced pressure.The reaction was poured into ice water with stirring, the aqueous phase was extracted with (3 × 50 ml) ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered and desolvated.The residue was separated by column chromatography (eluent was ethyl acetate and petroleum ether, volume ratio was 1: 5) to obtain a yellow liquid 14.43g, yield 88.5%. |
| 88.5% |
With trichlorophosphate; In toluene;Reflux; |
Dissolve 14.5g (0.1mol) of <strong>[7752-72-9]4-hydroxy-5-chloro-6-methylpyrimidine</strong> in 50ml of toluene solution, drop 50ml of phosphorus oxychloride into the reverse bottle with stirring, and after heating up, reflux and react for 5-7 hours , After the reaction was monitored by TLC. Toluene and excess phosphorus oxychloride were distilled off under reduced pressure, the reaction was poured into ice water with stirring, the aqueous phase was extracted with (3 × 50 ml) ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered and desolvated . Column chromatography of the residue (eluent is ethyl acetate and petroleum ether (boiling range 60-90 C), volume ratio is 1: 5) to obtain a yellow liquid 14.43g, yield 88.5%. |
|
With trichlorophosphate; In toluene;Reflux; |
General procedure: POCl3 (100 mL) was added dropwise to a solution of M-2 (0.36 mol)in toluene (150 mL), the mixture was refluxed for 3-5 h. The reactionmixture was concentrated under reduced pressure to remove tolueneand extra POCl3, and then poured into ice water. The water phase wasextracted with ethyl acetate (3 × 50 mL), the emerged organic phasewas successively washed with saturated sodium bicarbonate, dried overanhydrous magnesium sulfate, filtered and then concentrated underreduced pressure. The residue was purified through silica column togive M-3 as yellow liquid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping