| 64% |
With hydrogenchloride; In methanol; at 20℃; for 168h; |
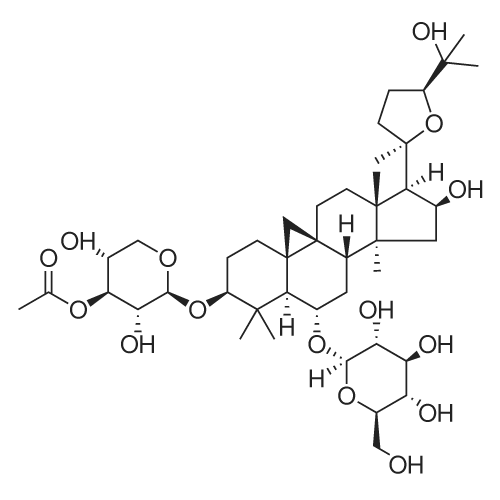
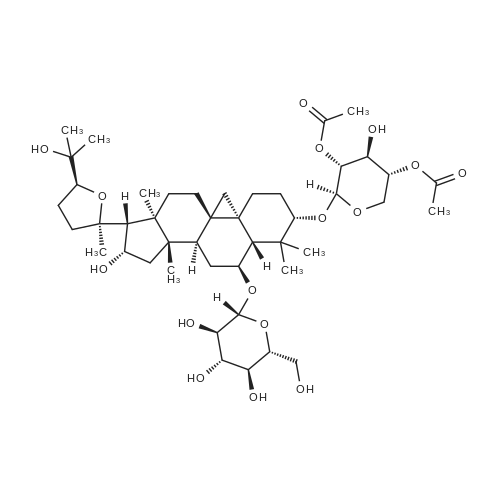
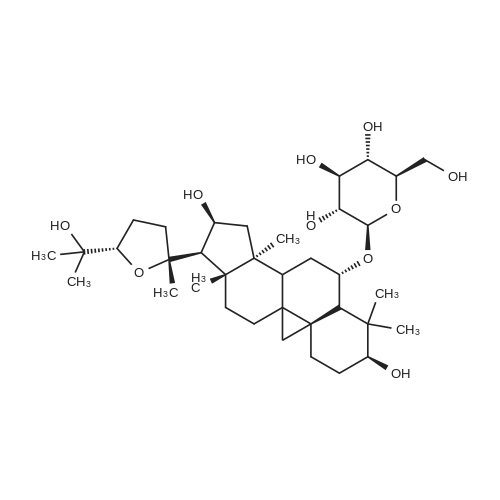
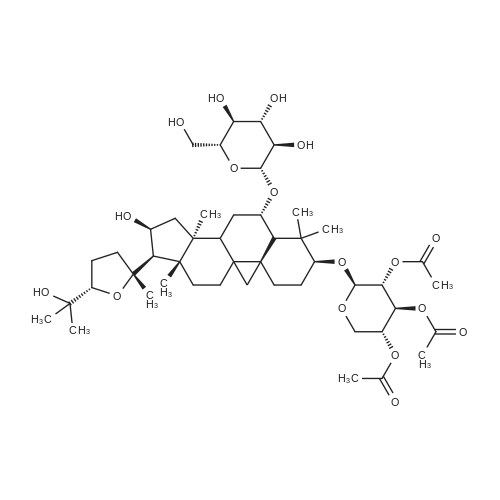
To <strong>[83207-58-3]astragaloside IV</strong> (1) (5.00 g, mmol) was added ?HCl-MeOH 10? (TCI America) (500 mL) and the mixture was stirred at room temperature for 7 days. The reaction mixture was concentrated to about half volume under reduced pressure at 20 C. (do not heat). The mixture was partitioned into aqueous sodium bicarbonate and ethyl acetate. The aqueous layer was extracted with ethyl acetate again. The organic layers were combined, washed with saturated sodium chloride, dried on anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by column chromatography (20:114:1 chloroform/methanol). In order to replace the residual solvent with ethanol, the purified material was dissolved in ethanol and the solvent was removed under reduced pressure to afford 2 (2.1 g, 64%).1H NMR (CDCl3) delta (ppm) 0.34 (d, J=4.7 Hz, 1H), 0.48 (d, J=4.3 Hz, 1H), 0.92 (s, 3H), 0.93 (s, 3H), 1.0-1.8 (m, 13H), 1.11 (s, 3H), 1.19 (s, 3H), 1.22 (s, 6H), 1.27 (s, 3H), 1.9-2.0 (m, 4H), 2.30 (d, J=7.8 Hz, 1H), 2.54 (q, J=11.8 Hz, 1H), 3.27 (m, 1H), 3.50 (m, 1H), 3.72 (t, J=7.4 Hz, 1H), 4.65 (q, J=7.4 Hz, 1H). ESI-MS m/z Positive 491 (M+H)+, Negative 549 (M+AcO)-. TLC (Merck, Kieselgel 60) Rf=0.33 (6:1 chloroform/methanol) |
| 64% |
With hydrogenchloride; In methanol; at 20℃; for 168h; |
To <strong>[83207-58-3]astragaloside IV</strong> (1) (5.00 g, mmol) was added ?HCl-MeOH 10? (TCI America) (500 mL) and the mixture was stirred at room temperature for 7 days. The reaction mixture was concentrated to about half volume under reduced pressure at 20 C. (do not heat). The mixture was partitioned into aqueous sodium bicarbonate and ethyl acetate. The aqueous layer was extracted with ethyl acetate again. The organic layers were combined, washed with saturated sodium chloride, dried on anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by column chromatography (20:114:1 chloroform/methanol). In order to replace the residual solvent with ethanol, the purified material was dissolved in ethanol and the solvent was removed under reduced pressure to afford 2 (2.1 g, 64%). (0173) 1H NMR (CDCl3) delta (ppm) 0.34 (d, J=4.7 Hz, 1H), 0.48 (d, J=4.3 Hz, 1H), 0.92 (s, 3H), 0.93 (s, 3H), 1.0-1.8 (m, 13H), 1.11 (s, 3H), 1.19 (s, 3H), 1.22 (s, 6H), 1.27 (s, 3H), 1.9-2.0 (m, 4H), 2.30 (d, J=7.8 Hz, 1H), 2.54 (q, J=11.8 Hz, 1H), 3.27 (m, 1H), 3.50 (m, 1H), 3.72 (t, J=7.4 Hz, 1H), 4.65 (q, J=7.4 Hz, 1H). ESI-MS m/z Positive 491 (M+H)+, Negative 549 (M+AcO)-. TLC (Merck, Kieselgel 60) Rf=0.33 (6:1 chloroform/methanol). |
|
With hydrogenchloride; In methanol; chloroform; water; at 20℃; for 144h; |
400 mg of <strong>[83207-58-3]astragaloside IV</strong> was taken as a raw material, and added to 400 ml of a 20% methanol aqueous solution having a volume fraction of 18% hydrochloric acid, and 640 ml of an organic phase chloroform was added.Stir vigorously for 6 days at room temperature, stand still after reaction, collect organic phase chloroform,The aqueous acid phase is neutralized by adding sodium hydrogencarbonate, and then an equal volume of chloroform is added for extraction.The combined organic phases were washed with a saturated aqueous solution of sodium chloride and dried over anhydrous sodium sulfate.Concentration under reduced pressure gave a crude material. The final cycloastragenol yield was calculated to be 46.28% by HPLC. Separation of cycloxanthine:200 mg of the above crude product was added to 600 mg of 200-300 mesh silica gel, and 8000 mg of silica gel was taken. The column was filled with chloroform in a wet manner, and the crude product mixed with silica gel was evenly dispersed on the top of the silica gel column, and then the volume ratio of chloroform:methanol was used. Elution was carried out at 15:1 with a flow rate of approximately 1.0 ml/min. The test was performed by thin layer chromatography every 10 ml of sampling. The eluent was collected and distilled under reduced pressure to give the product as a cyclohexanol having a purity of 99%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping