| 100% |
With ammonia hydrochloride; zinc powder; In tetrahydrofuran; methanol; at 40℃; for 1.0h; |
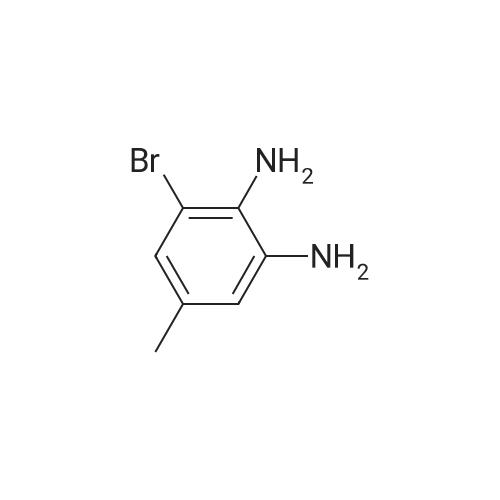
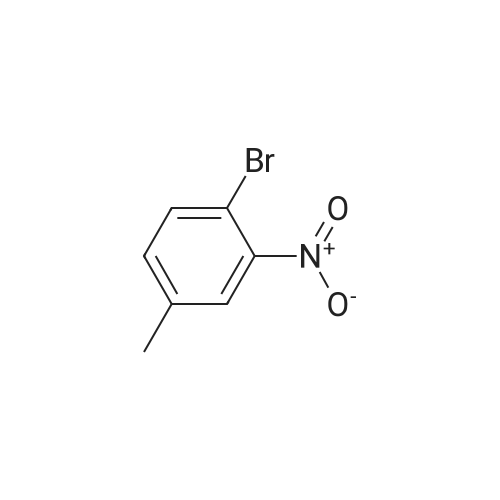
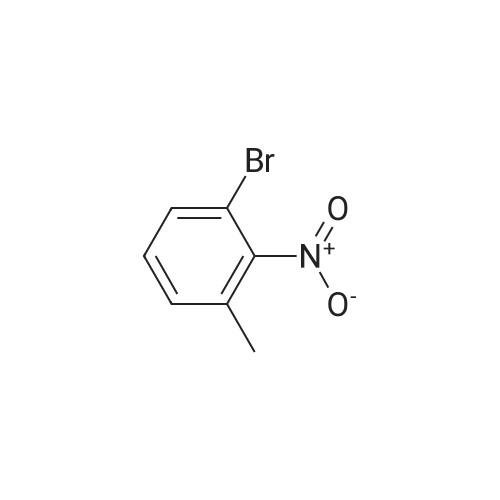
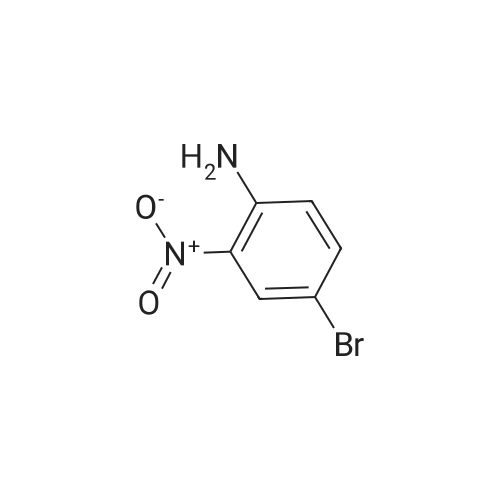
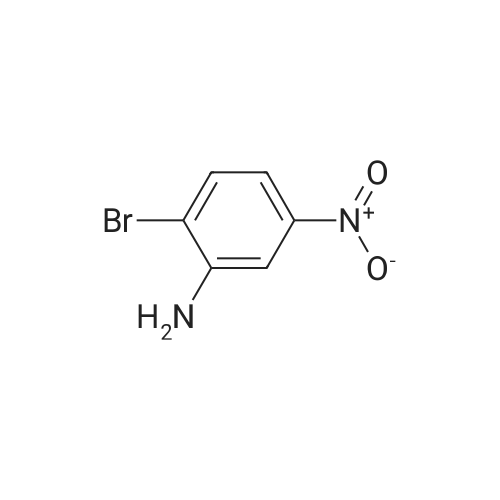
2-bromo-4-methyl-6-nitroaniline (5.00 g, 21.64 mmol) was dissolved in MeOH(148 mL) and THF (18.50 mL). Ammonium chloride (23.15 g, 433 mmol) then zinc (14.15 g, 216 mmol) were added and the reaction mixture was heated to 40 C for lh. The reaction mixture was cooled to ambient temperature, concentrated in vacuo, re-dissolved in EtOAc and saturated Na2CO3, and stirred vigorously for 10 minutes. The mixture was filtered through a sintered glass funnel and washed with more EtOAc. Theorganic layer was further washed twice with water, washed with brine, dried with sodium sulfate, filtered, and concentrated in vacuo to yield Intermediate I-iSA (4.35 g, 21.63 mmol, 100 % yield). ‘H NMR (400MHz, CHLOROFORM-d) 6.81 (s, 1H), 6.48 (s, 1H), 3.66 (br. s., 2H), 3.46 (br. s., 2H), 2.19 (s, 3H). LC-MS: method H, RT = 0.93 mm, MS (ESI) m/z: 201.0 (M+H) |
| 100% |
With ammonia hydrochloride; zinc powder; In tetrahydrofuran; methanol; at 40℃; for 1.0h; |
2-bromo-4-methyl-6-nitroaniline (5.00 g, 21.64 mmol) was dissolved in MeOH (148 mL) and THF (18.50 mL). Ammonium chloride (23.15 g, 433 mmol) then zinc (14.15 g, 216 mmol) were added and the reaction mixture was heated to 40 C for lh. The reaction mixture was cooled to ambient temperature, concentrated in vacuo, re-dissolved in EtOAc and saturated Na2CO3, and stirred vigorously for 10 minutes. Themixture was filtered through a sintered glass funnel and washed with more EtOAc. The organic layer was further washed twice with water, washed with brine, dried with sodium sulfate, filtered, and concentrated in vacuo to yield Intermediate I-iSA (4.35 g, 21.63 mmol, 100 % yield). ‘H NMR (400MHz, CHLOROFORM-d) 6.81 (s, 1H), 6.48 (s, 1H), 3.66 (br. s., 2H), 3.46 (br. s., 2H), 2.19 (s, 3H). LC-MS: method H, RT = 0.93 mm,MS (ESI) m/z: 201.0 (M+H) |
| 97% |
With hydrogenchloride; stannous chloride; In ethanol; water monomer; at 60℃; for 3.0h;Inert atmosphere; |
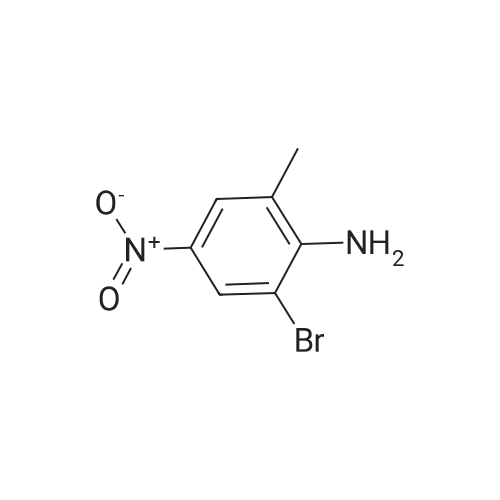
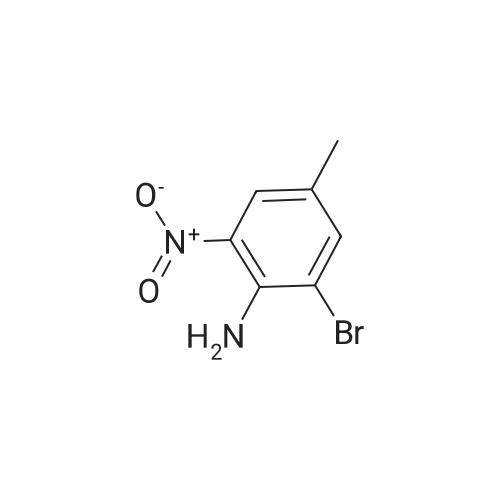
To a solution of Example 35a (20.0 g, 86.6 mmol, 1.0 eq) in EtOH (120 mL) and conc. HCl (40 mL) was added SnCl2 (97.4 g, 433 mmol, 5.0 eq). The reaction mixture was stirred at 60C for 3 h under N2. After cooled to room temperature, the mixture was poured into 2M NaOH aqueous solution (750 mL) at 0C. DCM (800 mL) was added to the mixture, and the white solid was removed by filtration. The organic layer was separated, and the aqueous phase was extracted with DCM (500 mL*2). The combined organic extracts were washed with brine, dried over Na2SO4, and concentrated. The crude was purified by silica gel flash column chromatography to afford the product Example 35b (16.8 g, 97% yield) as a yellow solid. LCMS [M+1]+ = 201.2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping