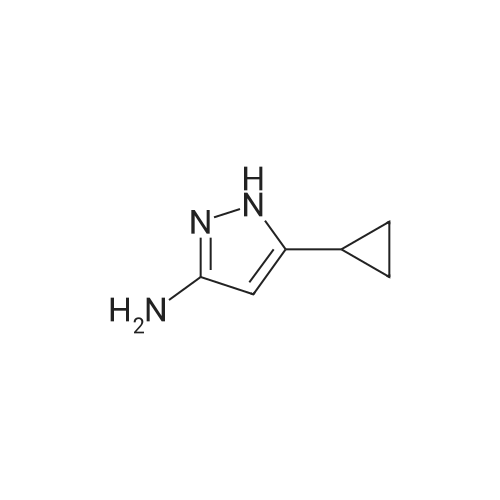
| 61% |
With copper(l) iodide; potassium carbonate; (1S,2S)-N,N'-dimethyl-1,2-diaminocyclohexane; In 5,5-dimethyl-1,3-cyclohexadiene; for 3h;Inert atmosphere; Reflux; |
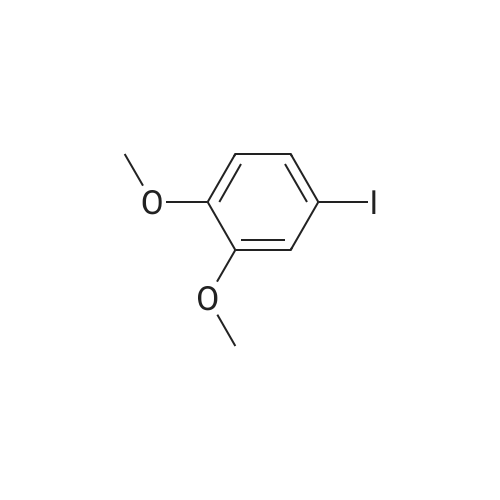
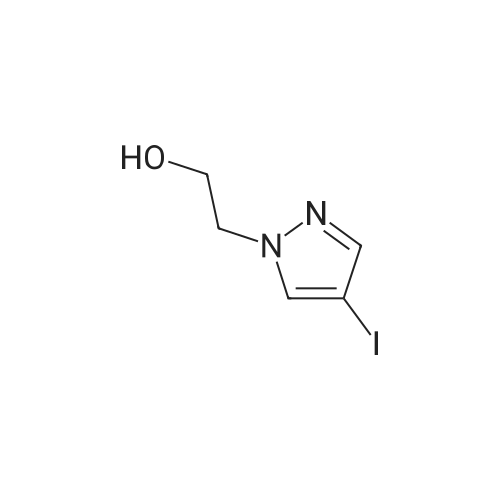
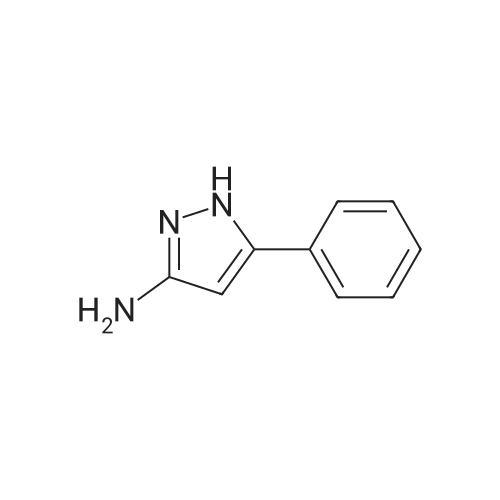
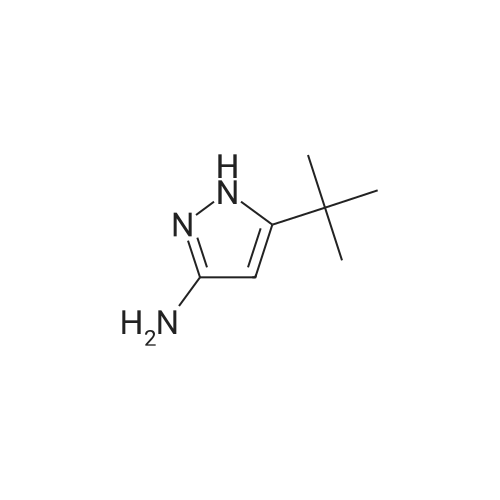
b. 2-(5-Amino-3-tert-butyl-[1,4']bipyrazolyl-1'-yl)-ethanol (Intermediate Gb) A solution of Intermediate Ga (9.36 g, 39.3 mmol) in xylene (40 mL) was purged with argon for 30 min. In a separate flask, a mixture of 3-tert-butyl-1H-pyrazole-5-amine (5.75 g, 41.3 mmol), copper iodide (375 mg, 1.97 mmol), trans-N,N-dimethylcyclohexane-1,2-diamine (1.12 g, 7.87 mmol) and potassium carbonate (11.4 g, 82.6 mmol) was de-gassed and purged with argon three times. The xylene solution was then added, via cannula to the flask and the resultant brown solution was heated at reflux for 3 h. The cooled solution was diluted with EtOAc (40 mL) and washed with saturated aqueous ammonia solution/water (1:1, 40 mL). The aqueous layer was extracted with EtOAc (40 mL) and the combined organics were washed with water (40 mL) and brine (40 mL), dried (Na2SO4), filtered and concentrated in vacuo to afford a solid. This was purified by FCC, using 4-7.5% MeOH in DCM to afford the title compound (6.01 g, 61%). LCMS (Method 1): Rt 1.84 min, m/z 250 [MH+]. |
| 61% |
With trans-N,N'-dimethyl-1,2-cyclohexyldiamine; copper(l) iodide; potassium carbonate; In 5,5-dimethyl-1,3-cyclohexadiene; for 3h;Inert atmosphere; Reflux; |
A solution of Intermediate 6a (9.36 g, 39.3 mmol) in xylene (40 mL) was purged with Argon for 30 min. In a separate flask, a mixture of 3-tert- butyl-lH-pyrazole-5-amine (5.75 g, 41.3 mmol), copper iodide (375 mg, 1.97 mmol), trans-N,N-dimethylcyclohexane- l ,2-diamine (1.12 g, 7.87 mmol) and potassium carbonate (1 1.4 g, 82.6 mmol) was de-gassed and purged with Argon three times. The xylene solution was then added, via cannula to the flask and the resultant brown solution was heated at reflux for 3 h. The cooled solution was diluted with EtOAc (40 mL) and washed with saturated aqueous ammonia solution/water (1 : 1 , 40 mL). The aqueous layer was extracted with EtOAc (40 mL) and the combined organics were washed with water (40 mL) and brine (40 mL), dried (Na2SO4), filtered and concentrated in vacuo to afford a solid. This was purified by FCC, using 4-7.5% MeOH in DCM, to afford the title compound (6.01 g, 61 %). LCMS (Method 3): Rt 1.84 min, m/z 250 [MH+]. |
| 61% |
With copper(l) iodide; potassium carbonate; (1S,2S)-N,N'-dimethyl-1,2-diaminocyclohexane; In 5,5-dimethyl-1,3-cyclohexadiene; for 3h;Inert atmosphere; Reflux; |
A solution of Intermediate Ga (9.36 g, 39.3 mmol) in xylene (40 mL) was purged with argon for 30 min. In a separate flask, a mixture of 3-tert-butyl-lH- pyrazole-5-amine (5.75 g, 41.3 mmol), copper iodide (375 mg, 1.97 mmol), trans- N,N-dimethylcyclohexane-l ,2-diamine (1.12 g, 7.87 mmol) and potassium carbonate (1 1.4 g, 82.6 mmol) was de-gassed and purged with argon three times. The xylene solution was then added, via cannula to the flask and the resultant brown solution was heated at reflux for 3 h. The cooled solution was diluted with EtOAc (40 mL) and washed with saturated aqueous ammonia solution/water (1 : 1 , 40 mL). The aqueous layer was extracted with EtOAc (40 mL) and the combined organics were washed with water (40 mL) and brine (40 mL), dried (Na2S04), filtered and concentrated in vacuo to afford a solid. This was purified by FCC, using 4-7.5% MeOH in DCM to afford the title compound (6.01 g, 61 %). LCMS (Method 1): Rt 1.84 min, m/z 250 [MH+]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping