| 61% |
|
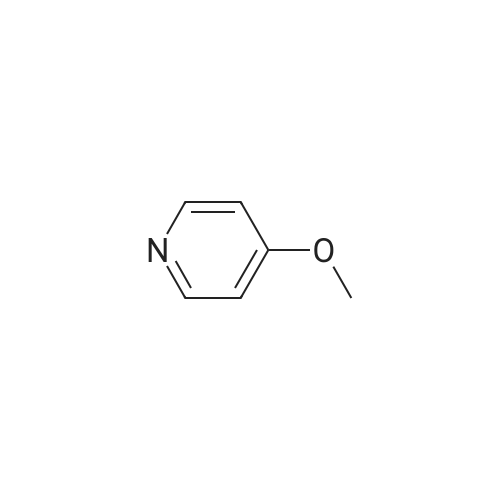
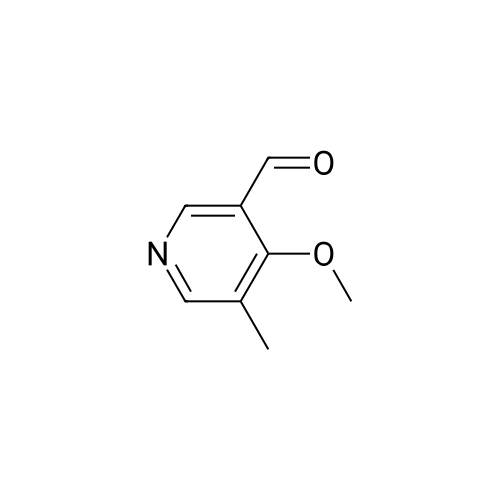
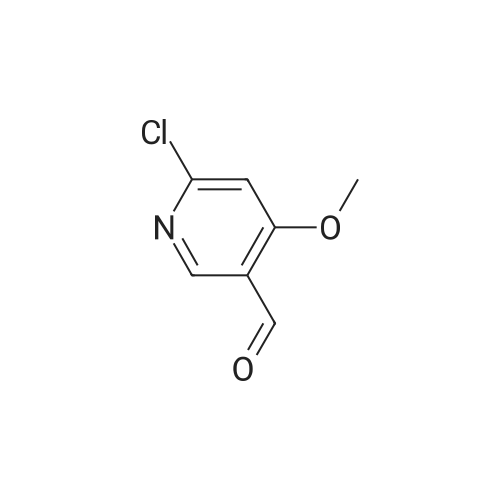
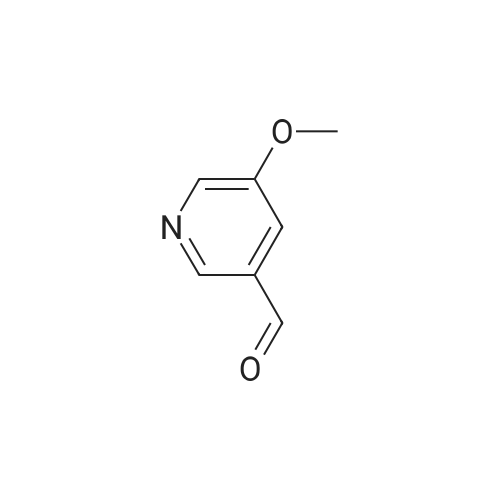
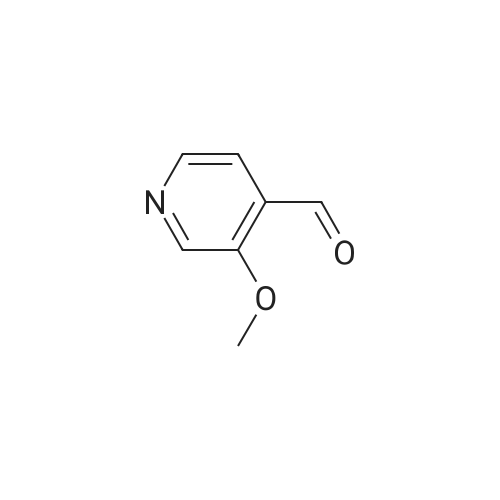
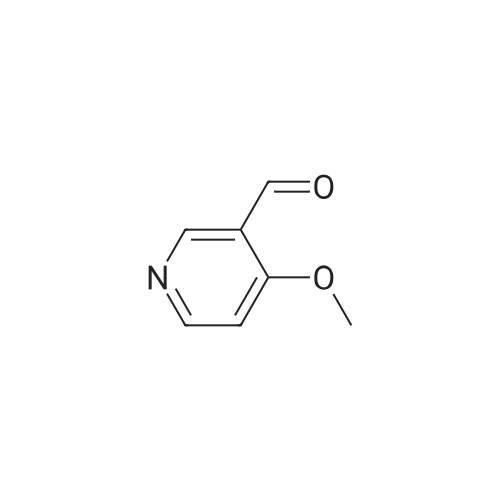
Example 2 Preparation of 4-Methoxypyridine-3-carboxaldehyde tert-Butyllithium (90.6 mL, 154 mmol ; 1.7 M in pentane) was added via cannula to a stirred solution of tetrahydrofuran (380 mL) under an atmosphere of nitrogen at room temperature. The reaction mixture was cooled to-78 C before adding 2-bromomesitylene (11.3 mL, 74.1 mmol) dropwise. The reaction mixture was allowed to stir for 1 hour at-78 C. To the reaction mixture at-78 C was added 4- methoxypyridine (5.79 mL, 57 mmol) dropwise, and the resulting mixture was stirred at- 23 C for 3 hours. The reaction mixture was then re-cooled to-78 C and dimethylformamide (6.62 mL, 85.5 mmol) was added and stirring was continued for 1 hour at-78 C. The reaction mixture was quenched slowly at-78 C with saturated aqueous sodium chloride solution (100 mL) and allowed to warm to room temperature slowly. To the reaction mixture was added diethyl ether (200 rnL) and the layers were separated. The aqueous layer was extracted with diethyl ether (2 x 150 mL) and the combined organic layers were dried over potassium carbonate (20 g). The potassium carbonate was removed by filtration and washed with diethyl ether (100 mL) and the solvent removed under reduced pressure. The resulting crude 4-methoxy-3- pyridinecarboxaldehyde was purified by column chromatography (SiO2, 5: 95 ethanol: ethyl acetate) to give 4.79 g of the title intermediate as a yellow solid (61 % yield; >98% purity by'H NMR). |
| 47.6% |
|
To a solution of t-butyllithium in THF (1.7 M, 9.7 mL, 16.5 mmol, 2.1 eq) in THF (40 mL) maintained at -78 C was added 2-bromomesitylene (1.6 g, 8.0 mmol, 1.3 eq) dropwise. The resulting solution was stirred for 1 h at -78 C, then 4-methoxypyridine (681 mg, 0.63 mL, 6.2 mmol) was added and the mixture was warmed to -20 C in an ice-salt bath. After stirring for 3 h at -20 C, the mixture was cooled back to -78 C and DMF (1.17 g, 16.0 mmol, 2.0 eq) was added. The reaction mixture was stirred for 1 h, quenched with brine at -78 C and extracted with diethyl ether (3 × 100 mL). The combined organic extracts were dried over K2CO3, filtered and evaporated to yield a crude oil that was purified by silica gel chromatography using 1:1 EtOAc:hexanes to yield 405 mg (47.6%) of product as a pale yellow oil: Rf 0.25 (1:1 hexane:EtOAc); 1H NMR (300 MHz, CDCl3) δ 10.43 (s, 1H), 8.87 (s, 1H), 8.62 (d, 1H), 6.92 (d, 1H), 4.1 (s, 3H). |
|
|
A flask is charged with 1.7M tert-butyllithium in pentane (47.1 ml_, 80.1 mmol) and THF (20 ml_), and cooled to -78 0C. 2-Bromomesitylene (6.0 ml_, 39 mmol) is added dropwise. The mixture is stirred for 1 h, and 4-methoxypyridine (3.0 ml_, 30 mmol) is added dropwise. The mixture is warmed to -23 C and stirred for 3 h. The mixture is <n="128"/>cooled again to -78 C and dimethylformamide (3.5 ml_, 45 mmol) is added. After 1 h, brine (50 ml.) is added to the mixture at -78 C and warmed to room temperature. The mixture is extracted with ether and the combined organic layer is dried over Na2SO4. Concentration followed by silica gel chromatography eluting with a O to 6% methanol- dichloromethane gradient gives 4-methoxy-pyridine-3-carbaldehyde. 1H NMR (400 MHz, CDCI3) δ ppm 4.01 (s, 3 H), 6.94 (d, J=5.8 Hz, 1 H), 8.65 (d, J=5.8 Hz, 1 H), 8.90 (s, 1 H), 10.46 (s, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping