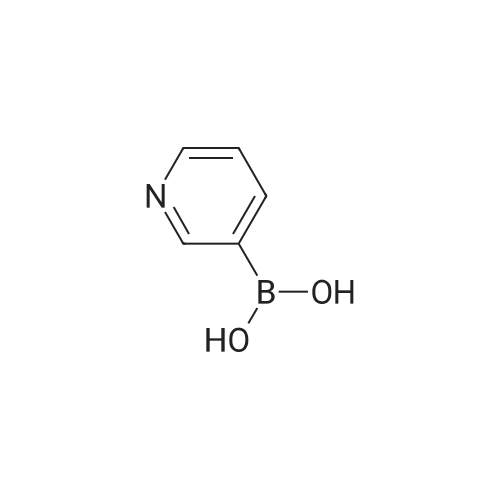
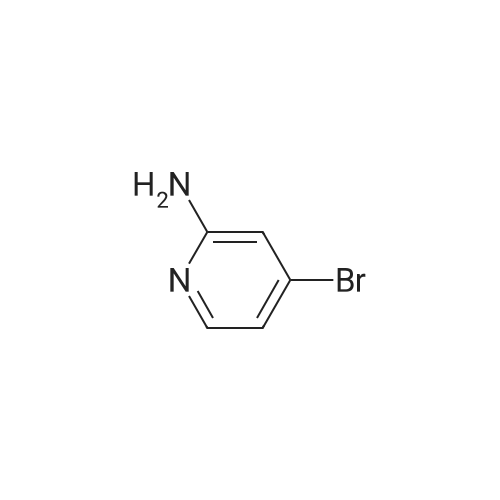
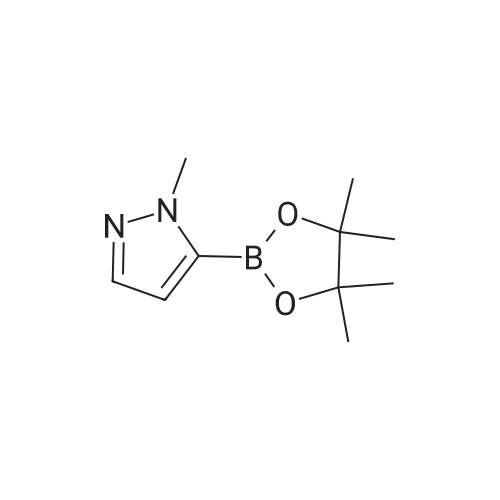
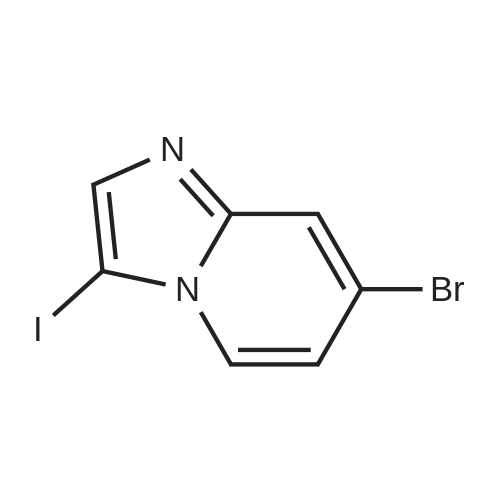
| 71% |
|
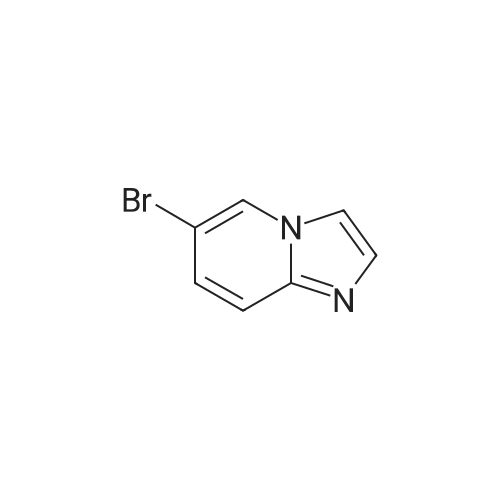
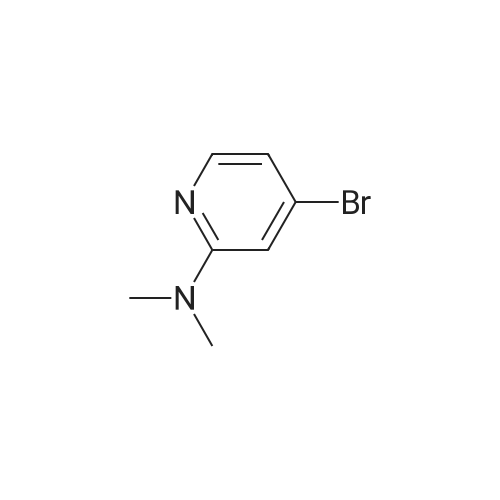
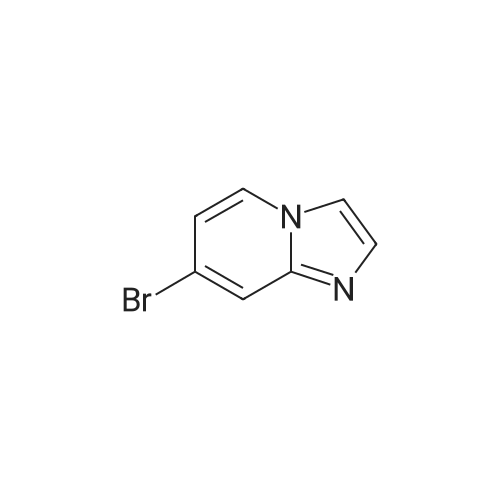
Example 44; 8-(ftrans)-3-fluoropiperidin-4-yloxy)-2-(7-(pyridin-3-yl)imidazo[1.2-a]pyridin-3-yl)qiotaiotainoluie; Step A: Preparation of 7-bromoimidazo[1.2-a]pyridine:; A solution of 4- bromopyridin-2-amine (1.00 g, 5.78 mmol) and 2-chloroacetaldehyde (50% wt aqueous solution, 1.83 ml5 14.45 mmol) in absolute ethanol (9.5 ml) was refluxed for 12 hours, and then allowed to cool to ambient temperature overnight. The reaction mixture was concentrated under reduced pressure and carefully re-suspended in saturated aqueous bicarbonate solution (100 ml). The resulting mixture was extracted thoroughly with DCM and EtOAc, and the combined organic extracts were dried over anhydrous sodium sulfate and concentrated to afford 1.31 g of a solid. The solid was purified by silica gel chromatography (eluting with 3% MeOH-chloroform) to afford the desired compound (0.808 g5 71 % yield). MS APCI (+) m/z 197.1 and 199.1 (M+l for each isotope) detected. |
| 71% |
In ethanol; water; at 20℃; for 12h;Heating / reflux; |
A solution of 4- bromopyridin-2-amine (1.00 g, 5.78 mmol) and 2-chloroacetaldehyde (50% wt aqueous solution, 1.83 ml, 14.45 mmol) in absolute ethanol (9.5 ml) was refluxed for 12 hours, and then allowed to cool to ambient temperature overnight. The reaction mixture was concentrated under reduced pressure and carefully re-suspended in saturated aqueous bicarbonate solution (100 ml). The resulting mixture was extracted thoroughly with DCM and EtOAc, and the combined organic extracts were dried over anhydrous sodium sulfate and concentrated to afford 1.31 g of a solid. The solid was purified by silica gel chromatography (eluting with 3% MeOH-chloroform) to afford the desired compound (0.808 g, 71 % yield). MS APCI (+) m/z 197.1 and 199.1 (M+l for each isotope) detected. |
| 63% |
With sodium hydrogencarbonate; In ethanol; for 16h;Reflux; |
To a stirred solution of 4-bromopyridin-2-amine (5 g, 28.9 mmol, Molekula Biokem Ltd) in EtCH (5OmL), added sodium bicarbonate (7.28 g, 86.7 mmol) and chloroacetaldehyde (5 mL, 115 mmol) and refluxed for 16 h. The reaction mixture was evaporated under vacuum and water (25 mL) was added to the crude mixture. The resulting solution was extractedwith EtOAc (2 x 50 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated. The resulting crude product was purified by flash chromatography. Yield:63% (3.6 g, brown solid). LCMS: (Method B) 199.0 (M +H), Rt. 3.92 mm, 94.50% (Max). |
| 63% |
With sodium hydrogencarbonate; In ethanol; for 16h;Reflux; |
To a stirred solution of 4-bromopyridin-2-amine (5 g, 28.9 mmol, Molekula Biokem Ltd) in EtOH (50ml_), added sodium bicarbonate (7.28 g, 86.7 mmol) and chloroacetaldehyde (5 mL, 1 15 mmol) and refluxed for 16 h. The reaction mixture was evaporated under vacuum and water (25 mL) was added to the crude mixture. The resulting solution was extracted with EtOAc (2 x 50 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated. The resulting crude product was purified by flash chromatography. Yield: 63% (3.6 g, brown solid). LCMS: (Method B) 199.0 (M +H), Rt. 3.92 min, 94.50% (Max). |
|
With sodium hydrogencarbonate; In ethanol; for 17h;Heating / reflux; |
4-Bromo-pyridin-2-ylamine (1 eq, 5.78 mmol, 1 g) is added to a solution of chloroacetic aldehyde (5 eq, 28.9 mmol, 5 ml) in EtOH (25 ml). NaHCO3 (2 eq, 11.6 mmol, 971 g) is then added and the reaction mixture is heated at reflux for 17 h. The solvent is then removed in vacuo and the product is purified by flash column chromatography eluting with 9:1 DCM/MeOH to afford 7-bromo-imidazo-[1,2-a]- pyridine as a brown solid; [M+H]+ = 198 |
|
|
INTERMEDIATE SYNTHESIS; 7-bromo-3-iodoimidazo[ 1 ,2-a]pyridine; (1) 7-bromoimidazo[l,2-a]pyridine.; To a 100 niL round -bottomed flask was added 4-bromopyridin-2-amine (4.0 g, 23.1 mmol), chloroacetaldehyde, 50% in water (14.9 rnL, 116 mmol), and EtOH (25 mL). The resulting reaction mixture was heated at 100 0C under N2 for 3 h. The reaction was cooled to rt and the solvent was concentrated. The residue was redissolved in EtOAc. The organic layer was washed with sat. NaHCObeta (2 x 40 mL), water (2 x 40 mL), brine, dried over MgSO4, and removed solvent. The crude product was purified using SiO2 chromatography (Teledyne Isco RediSep, 12O g SiO2, DCM:MeOH=96%:4% to DCM:MeOH (2M NH3)=95%:5%, Flow = 85 niL/min). The solvent was removed in vacuo to afford the desired product as brown solid (3.8 g). MS (ESI pos. ion) m/z: 196.8. Calcd exact mass for C7H5BrN2: 195.9. 1U NMR (300 MHz, CHLOROFORM-J) delta ppm 6.90 (d, J=7.16 Hz, 1 H) 7.57 (s, 1 H) 7.62 (s, 1 H) 7.83 (s, 1 H) 8.00 (d, J=7.16 Hz, 1 H). |
| 18.6 g |
In ethanol; water; at 0℃;Reflux; |
The compound 2-amino-4-bromopyridine 6 (20.00 g, 116.28 mmol) was dissolved in a mixture of ethanol (300 mL) and water (30 mL).After stirring for 20 minutes under ice bath conditions, when the solution temperature drops to 0 C,40% chloroacetaldehyde (28.60 mL, 174.40 mmol) was added slowly and stirred at reflux overnight.The mixture was filtered with Celite, and then evaporated and evaporated.Washed with water, washed with saturated brine, dried with Na2SO4 and then driedColumn chromatography gave a brown solid 8 (18.60 g, 81%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping