| 99% |
palladium-carbon; In ethanol; |
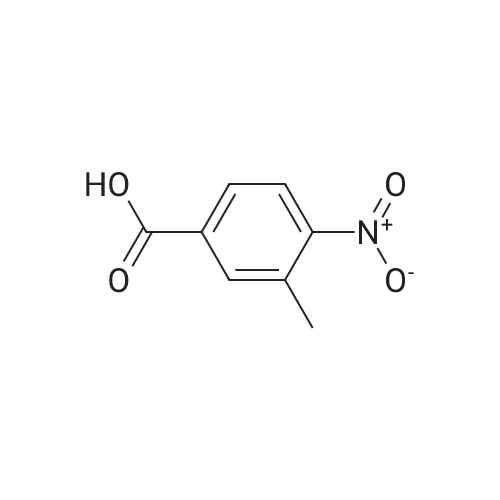
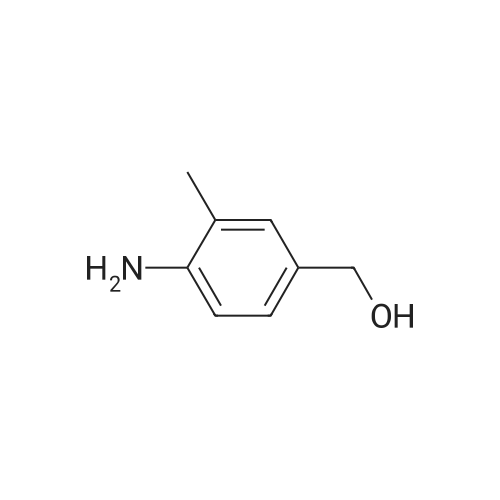
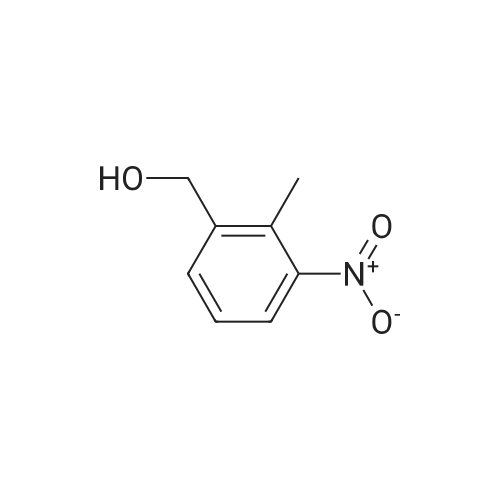
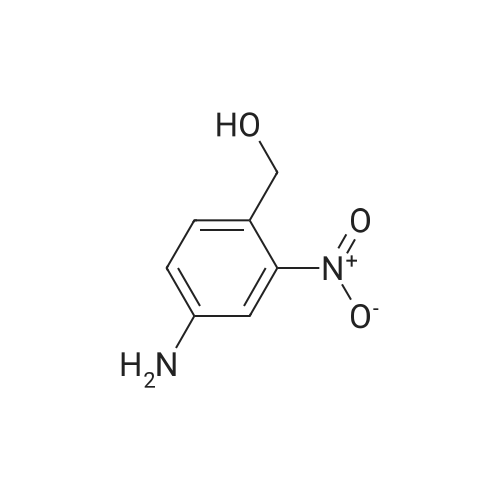
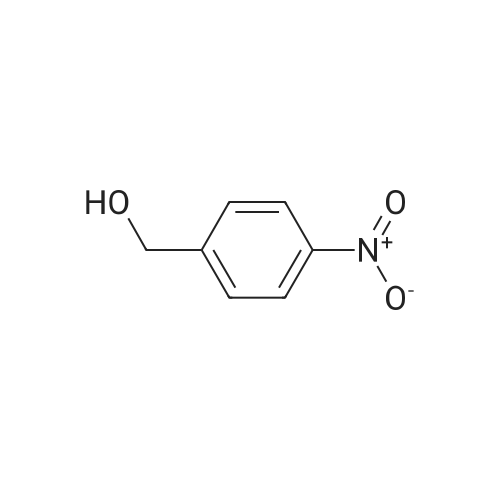
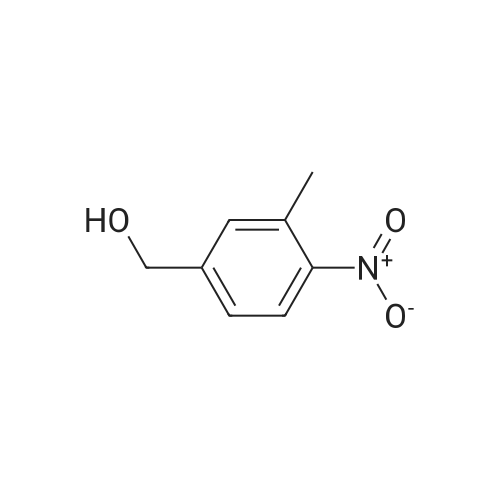
Example 79 Preparation of 4-(hydroxymethyl)-2-methylaniline (105) (Literature reference for making this compound see: Sun, J.-H.; Teleha, C. A.; Yan, J.-S.; Rodgers, J. D.; Nugiel, D. A. J. Org. Chem. 1997, 62, 5627-5629). A solution of <strong>[80866-75-7]3-methyl-4-nitrobenzyl alcohol</strong> (compound 104, 8.5 g, 50.9 mmol) in ethanol (100 mL) was stirred at room temperature, while 10% Pd/C (1.0 g) was added in one portion. The resulting suspension was hydrogenated (10 psi) in a Parr apparatus at room temperature for 1.5 hours. The catalyst was removed by filtration, and solvent was evaporated under vacuum to give the title compound (105, 6.9 g, 99%). MS (electrospray) 138 (M+1); 1H N MR (CDCl3) δ 2.16 (s, 3H), 2.72 (br s, 3H), 4.52 (s, 2H), 6.64 (d, 1H, J=8.0 Hz), 7.02 (d, 1H, J=8.0 Hz), 7.05 (s, 1H). Use of high pressure (30 Psi) of hydrogen and a long reaction time (8 hours) resulted a hydrogenolysis product. The same starting material (104) (21.05 g, 126 mmol) yielded 2,4-dimethylaniline (15.20 g, 100%) under such conditions. MS (electrospray) 122 (M+1); 1H N MR (CDCl3) δ 2.14 (s, 3H), 2.23 (s, 3H), 3.72 (br s, 1H), 6.58 (d, 1H, J=8.0 Hz), 6.84 (d, 1H, J=8.0 Hz), 6.87 (s, 1H). |
| 99% |
palladium-carbon; In ethanol; |
Example 68 Preparation of 4-(hydroxymethyl)-2-methylaniline (90) (Literature reference for making this compound see: Sun, J.-H.; Teleha, C. A.; Yan, J.-S.; Rodgers, J. D.; Nugiel, D. A. J. Org. Chem. 1997, 62, 5627-5629). A solution of <strong>[80866-75-7]3-methyl-4-nitrobenzyl alcohol</strong> (compound 89, 8.5 g, 50.9 mmol) in ethanol (100 mL) was stirred at room temperature, while 10% Pd/C (1.0 g) was added in one portion. The resulting suspension was hydrogenated (10 psi) in a Parr apparatus at room temperature for 1.5 hours. The catalyst was removed by filtration, and solvent was evaporated under vacuum to give the title compound (90, 6.9 g, 99%). MS (electrospray) 138 (M+1); 1H N MR (CDCl3) δ2.16 (s, 3H), 2.72 (br s, 3H), 4.52 (s, 2H), 6.64 (d, 1H, J=8.0 Hz), 7.02 (d, 1H, J=8.0 Hz), 7.05 (s, 1H). Use of high pressure (30 Psi) of hydrogen and a long reaction time (8 hours) resulted a hydrogenolysis product. The same starting material (89) (21.05 g, 126 mmol) yielded 2,4-dimethylaniline (15.20 g, 100%) under such conditions. MS (electrospray) 122 (M+1); 1H N MR (CDCl3) δ2.14 (s, 3H), 2.23 (s, 3H), 3.72 (br s, 1H), 6.58 (d, 1H, J=8.0 Hz), 6.84 (d, 1H, J=8.0 Hz), 6.87 (s, 1H). |
| 97% |
With hydrogen;palladium 10% on activated carbon; In ethanol; at 20℃; |
Example 4F1-(Tetrahydro-pyran-2-yl-1 H-indazo-5-yl}-methylamine: synthesized as described in the reference. JOC, 62, 5627(1997).268 269 270Part A:A mixture of 3-methyl -4-nitro benzyl alcohol 264 (2.10 g, 12.6 mmol) and 10% Palladium on carbon (0.2 g) in 25 mL of EtOH was hydrogenated at room temperature. After completion of the reaction, the catalyst was removed by filtration. <n="143"/>The solvent was evaporated and residue dried in a vacuum to give title compound as yellow solid 1.7 g, 97% ), 1H NMR (CDCI3), δ 7.06(s,1 H),7.03(d, J=8.0 Hz, 1 H), 6.66 (d, J=7.7 Hz, 1 H), 4.53 (s, 1 H), 3.62(br, 2H), 2.17 (s, 3H); mass calculated for compound 265 is 137.17, observed LCMS m/z 138.2 (M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping