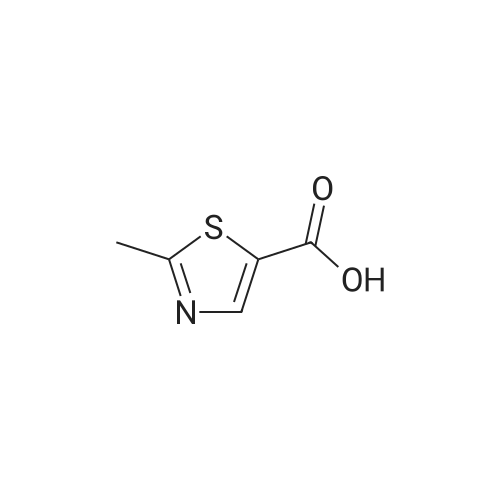
| 47% |
In benzene; for 4h;Reflux; Inert atmosphere; |
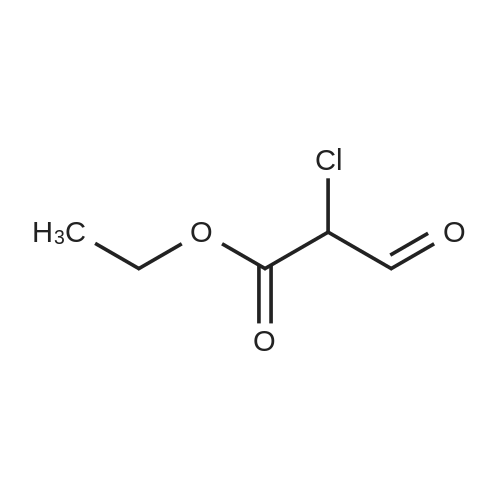
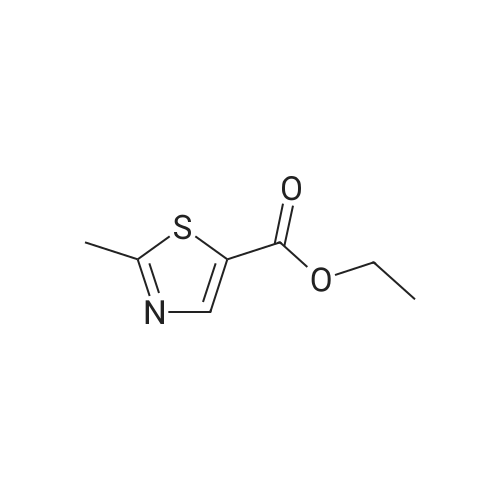
Example 72- [2-(5-Methyl-3-phenyl-isoxazol-4-yl)-ethyl] -thiazole-5-carboxylic acid isopropyl- amidea) 2-Methyl-thiazole-5-carboxylic acid ethyl esterTo a stirred solution of ethyl 2-chloro-2-formyl acetate (5.0 g, 33 mmol) in benzene (50 mL) at reflux under argon was added thioamide (2.5 g, 33 mmol). After 4 h the reaction mixture was cooled, diluted with water (50 mL) and neutralized to pH 7 with a saturated solution of sodium hydro gencarbonate. The reaction mixture was extracted with ethyl acetate then the combined extracts were washed with water and brine, then dried, filtered and concentrated in vacuo. Purification by chromatography (silica, 0 to 50percent ethyl acetate in heptane) gave the title compound (2.68 g, 47percent) as a yellow liquid. MS: m/e = 172.0 [M+H]+. |
| 35% |
With magnesium sulfate; In ethanol; for 24h;Inert atmosphere; Reflux; |
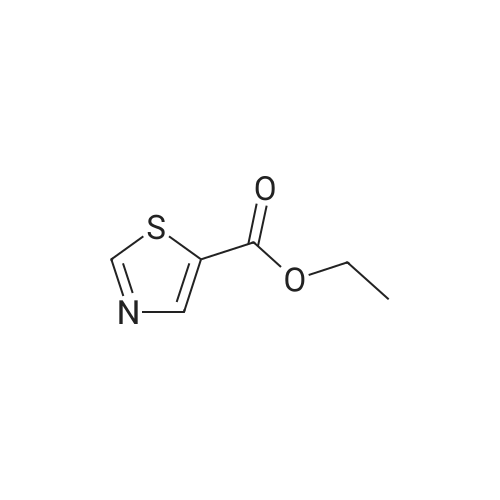
To a stirred solution of <strong>[33142-21-1]ethyl 2-chloro-3-oxopropanoate</strong> 61 (26 g, 173.33 mmol) in ethanol (200 mL) under argon atmosphere were added ethanethioamide 62 (10 g, 133.33 mmol), dry magnesium sulfate (10 g) at RT and heated to reflux for 24 h. The reaction was monitored by TLC; after completion of the reaction, the volatiles were removed in vacuo, diluted with EtOAc (500 mL). The combined organic extracts were washed with saturated sodium bicarbonatesolution (2 x 200 mL), brine (200 mL), dried over sodium sulfate, filtered and concentrated invacuo to obtain the crude. The crude was purified through flash column chromatography using6percent EtOAc/ hexanes to afford compound 63 (8 g, 35percent) as brown syrup. TLC: 25percent EtOAc/hexanes (Rf: 0.7); ?H-NMR (DMSO-d6, 500 MHz): oe 8.24 (s, 1H), 4.27 (q, J = 7.2 Hz, 2H),2.70 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H). |
| 33% |
With magnesium sulfate; In ethanol; at 80℃; for 16h;Inert atmosphere; |
To a stirring solution of compound 96 (110 g, 733.33 mmol) in ethanol (1.2 L) under Ar atmosphere were added ethanethioamide (54.99 g, 733.33 mmol) and anhydrous magnesium sulfate (55 mg, 454.66 mmol) at room temperature, followed by heating to 80 oC for 16 h. The reaction was monitored by TLC. After completion of the reaction, the volatiles were removed in vacuo. The residue was diluted with water and extracted using EtOAc. The organic extract was dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to obtain the crude. The crude was purified through silica gel column chromatography using 10percent EtOAc/hexanes to afford compound 97 (41 g, 33percent) as thick syrup. TLC: 20percent EtOAc/ hexanes (Rf: 0.3); 1H NMR (400 MHz, DMSO-d6): delta 8.26 (s, 1H), 4.29 (q, J = 7.2 Hz, 2H), 2.71 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H); LCMS Calculated for C7H9NO2S: 171.04; Observed: 172.1 (M+1)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping