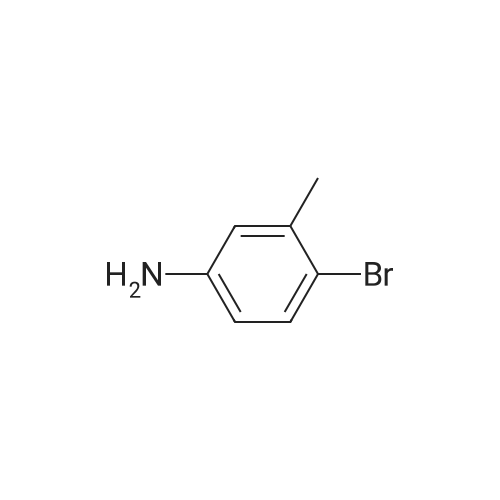
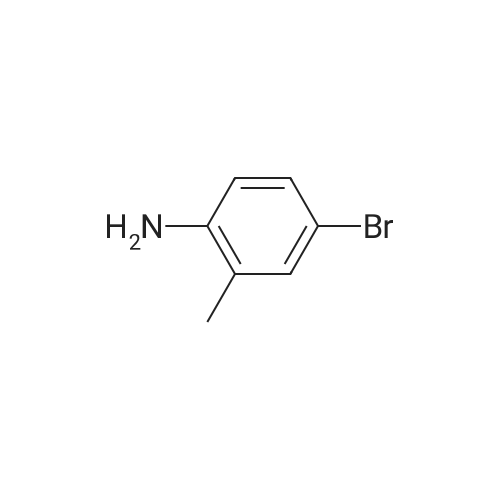
| 92.2% |
Stage #1: With hydrogenchloride In water at 90℃; for 1 h;
Stage #2: With sodium nitrite In water at -10 - 5℃; for 0.5 h;
Stage #3: With potassium iodide In water at 10 - 40℃; for 4 h; |
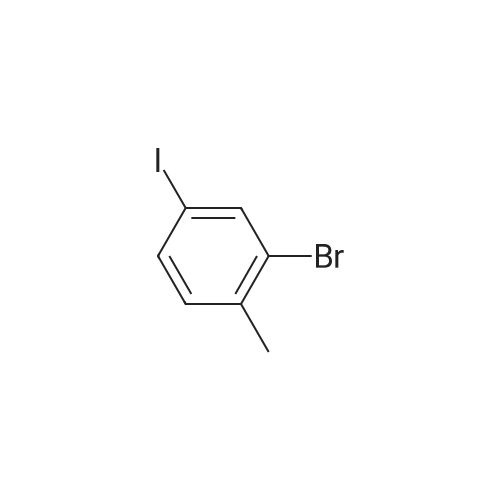
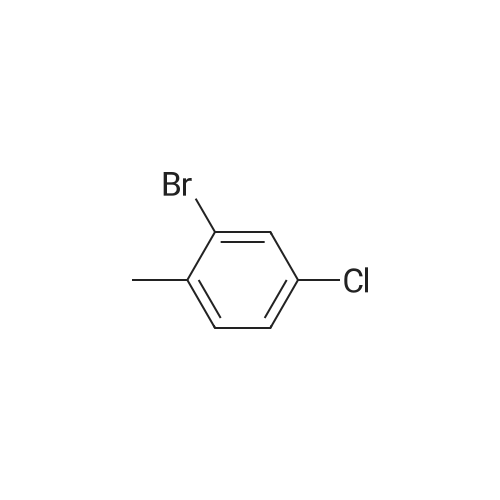
450 mL of water, 50.0 g (0.268 mol) of 2-bromo-p-toluidine,100 mL (1.102 mol) of concentrated hydrochloric acid was added and heated to 90 ° C. with stirring.Subsequently, the mixture was stirred at the same temperature for 1 hour, then cooled to room temperature, and further cooled to -10 ° C. with an ice water bath. Next, an aqueous solution prepared by dissolving 22.3 g (0.323 mol) of sodium nitrite in 70 mL of water was added dropwise at a temperature not exceeding 5 ° C., followed by stirring at 5 ° C. or lower for 30 minutes, filtration with an aid, diazonium An aqueous solution of salt was obtained.Next, 67.0 g (0.403 mol) of potassium iodide (Wako Pure Chemical Industries, Ltd.) and 230 mL of water were added to a 1 L four-necked round bottom flask equipped with a stirrer, Erlin condenser, 1 L dropping funnel and thermometer The mixture was placed in a water bath and stirred at 10 ° C. Next, after the aqueous solution of the diazonium salt was dropped, the water bath was removed to return to room temperature, the temperature was further increased to 40 ° C., and the mixture was stirred for 4 hours. The obtained reaction solution was returned to room temperature again, 300 mL of DCM was added and the mixture was transferred to a 2 L separatory funnel, then the aqueous layer was separated and extracted with 250 mL of DCM. Next, the DCM layers were combined, washed with 250 mL of 20percent sodium thiosulfate aqueous solution, 250 mL of saturated aqueous multilayer water, three times with 250 mL of water, dried with magnesium sulfate, then removed by suction filtration of magnesium sulfate, The solvent was distilled off under reduced pressure. Subsequently, the resulting crude oil was purified by silica gel column chromatography using n-heptane as a developing solvent to obtain 73.5 g (yield 92.2percent) of the desired iodide. |
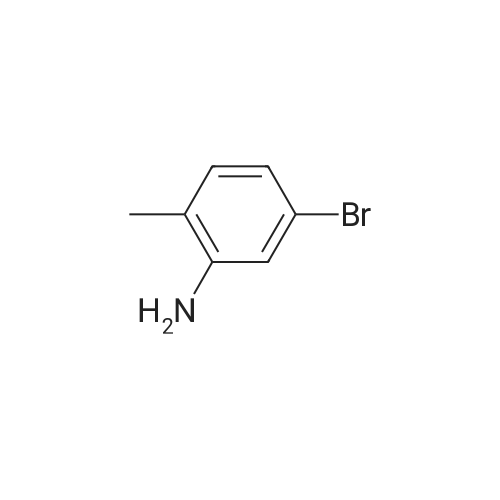
| 49% |
Stage #1: With hydrogenchloride; sodium nitrite In water at -8 - 0℃; for 0.75 h; Inert atmosphere
Stage #2: With potassium iodide In water at 0℃; for 3 h; Inert atmosphere |
(2-1) Synthesis of 2-Bromo-4-Iodotoluene (0143) A mixture of 3-bromo-4-methylaniline (18.6 g) and 6 M hydrochloric acid (80 mL) was cooled to ?5° C. in an argon atmosphere. After the dropwise addition of an aqueous solution (15 mL) of sodium nitrite (7.35 g) while maintaining the mixture at 0° C. or less, the resulting mixture was stirred at ?8° C. for 45 minutes. Potassium iodide (33.2 g) was added to the mixture over 3 hours while maintaining the mixture at 00° C. or less. The resulting reaction mixture was returned to room temperature. After the addition of a 10percent sodium hydrogen sulfite aqueous solution (50 mL), the mixture was extracted with diethyl ether. The organic layer was washed with a 10percent sodium hydrogen sulfite aqueous solution, and dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography to obtain 2-bromo-4-iodotoluene (14.5 g). The yield was 49percent. |
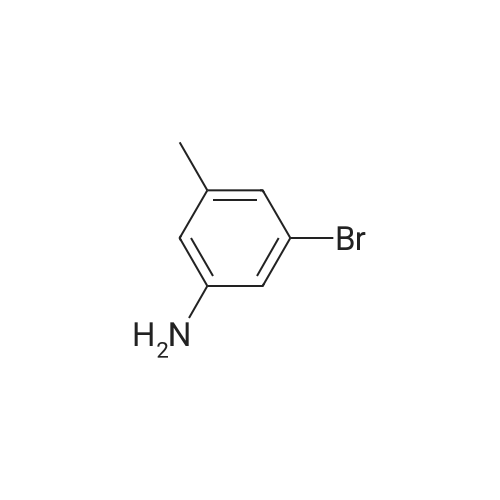
| 0.053 mol,53% |
With potassium iodide; sodium nitrite In hydrogenchloride; hexane; water |
Step A:
2-Bromo-4-iodotoluene
A well stirred solution of 18.6 g (0.10 mol) of 3-bromo-p-toluidine in 80 mL of 6N HCl at 0° C. was treated with a solution of 7.35 g (0.11 mol) of sodium nitrite in 15 mL of water at a rate that maintained the temperature <10° C.
The mixture was stirred for 45 minutes then cautiously treated with 33.2 g (0.20 mol) of potassium iodide at 0° C.
The mixture was treated with 300 mL of ether and washed (3*) with saturated aqueous sodium bisulfite.
The organic layer was separated, dried over magnesium sulfate, filtered and concentrated under vacuum.
The residue was redissolved in 50 mL of hexane, filtered through 30 g of silica and concentrated under vacuum to afford 15.6 g (0.053 mol,53percent) of the product which was determined to be 65percent pure by 1 H NMR. 1 H NMR (200 MHz,CDCl3):
2.33 (s,3H), 6.97 (d,8 Hz,1H), 7.51 (dd;2,8 Hz,1H), 7.86 (d,2 Hz,1H).
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping