| 94% |
With sodium hydroxide; In 1,4-dioxane; water; at 0℃; for 16h; |
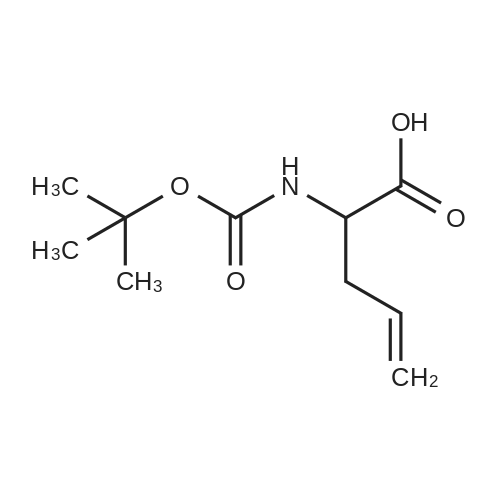
A solution of di-tert-butyl dicarbonate (11.4 g, 52.1 mmol) in 1,4-dioxane (15 mL) was slowly added to a second solution of 2-amino-5-pentenoic acid (5 g, 43.4 mmol) in 1 N NAOH (55 mL) at 0 C. After stirring for 16h, the solution was acidified with 5% HC1 to pH 2, and the resulting mixture was extracted with ethyl acetate (3 x 80 mL). The combined organic layers were dried OVER MGS04 and concentrated. The resulting residue was dissolved in DMF (50 mL) and treated with K2CO3 (7 g, 51 MMOL). After stirring for 15 min, the solution was cooled to 0 C and treated with iodomethane (3.4 mL, 51 mmol). After the addition was complete, the reaction mixture was stirred at room temperature for another 4 h, filtered and the resulting solid was washed with ethyl acetate (200 mL). The filtrate was washed successively with 5% aq HC1, sat. aq NaCl, dried over MGS04 and concented. Purification by flash column chromatography (5% ethyl acetate in heptane) provided 2-tert-Butoxycarbonylamino-5-pentenoic acid methyl ester (9.03 g, 94%) as an oil. 1H NMR (CDC13), 5.73 (m, 1 H), 5.12 (m, 1 H), 5.03 (m, 1 H), 4.38 (dd, J = 6, 12 Hz, 1 H), 3.74 (s, 3 H), 2.51 (m, 2 H), 1.46 (s, 9 H). |
| 93% |
|
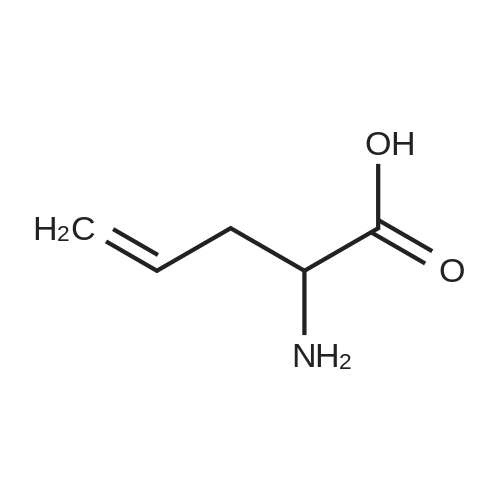
rac. )-2-tert-Butoxycarbonylamiiio-pent-4-enoic acid (A); A solution of DL-allylglycine (20.2 g; 175mmol) in THF/H2O (670/670 ml) was cooled to 0 0C. NaHCO3 (44.11g; 525mmol) was added portionwise and BoC2O (68.8g; 315mmol) was added also portionwise. The reaction mixture was stirred at 0 C under N2 for 5 min, then at rt overnight. The pH of the milky mixture was adjusted to 4 by carefully addition of saturated citric acid at 0C, and the mixture was extracted with EtOAc. The combined org. layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The title compound was isolated as a white solid (35.1 g, 93%). |
| 58% |
|
A mixture of 2-aminopent-4-enoic acid (230mg, 2mmol), water (3mL) and tert-butanol (1mL) was treated with NaOH (88mg, 2.2mmol) and stirred until a clear solution was obtained (2-5min). Then Boc-anhydride (654mg, 3mmol) in tert-butanol (1.3mL) was added and stirred at r.t. overnight. The reaction mixture was extracted with diethyl ether (2×25mL). The combined ethereal layer was extracted with saturated Na2CO3 (2×25mL) and the combined aqueous layer was acidified with conc. HCl to pH 1. The aqueous layer was extracted with diethyl ether (3×25mL). The combined organic extracts were washed with brine (25mL), dried (MgSO4) and the solvent was evaporated to give a light brownish oil. Yield: 250mg (58%). 1H NMR (CDCl3) δ 1.42 (s, 9H, CH3), 2.57 (br m, 2H, H-3), 4.22 (br m, 0.25 H, H-2), 4.39 (br m, 0.75 H, H-2), 5.10 (br m, 0.75 H, NH), 5.13 (s, 1H, H-5cis), 5.18 (d, 3J=9Hz, H-5trans), 5.74 (m, 1H, H-4), 6.11 (br m, 0.25 H, NH), 8.24 (br s, 1H, CO2H). 13C NMR (CDCl3) δ 28.3 (q, CH3), 36.4 (t, C-3), 52.8 (d, C-2), 80.3 (s, O-C), 119.3 (t, C-5), 132.2 (d, C-4), 155.5 (s, Boc), 176.2 (s, C-1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping