| 98.7% |
|
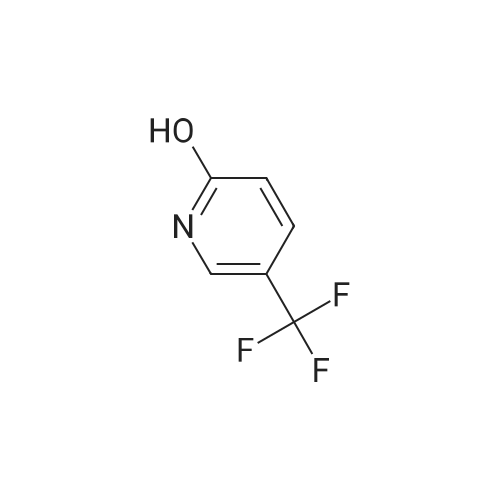
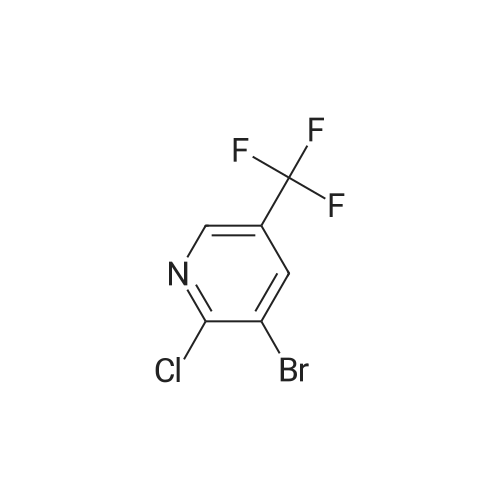
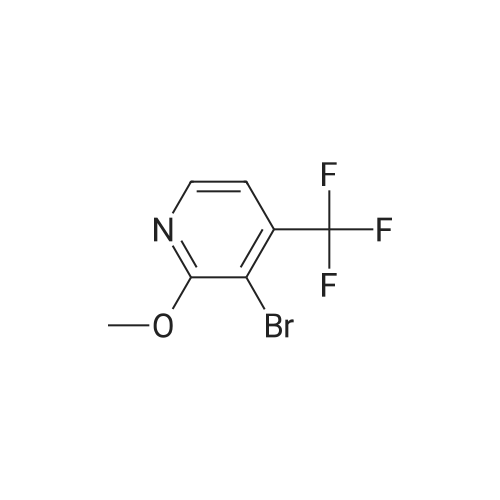
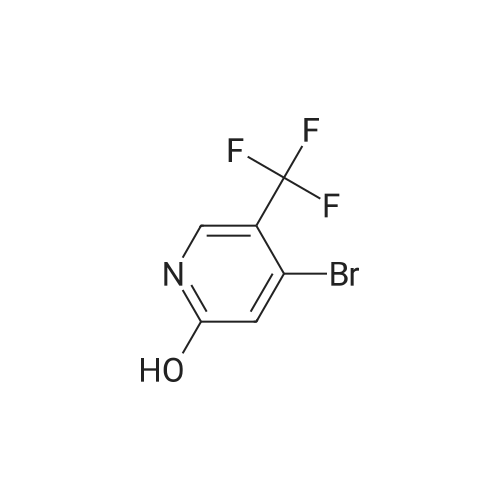
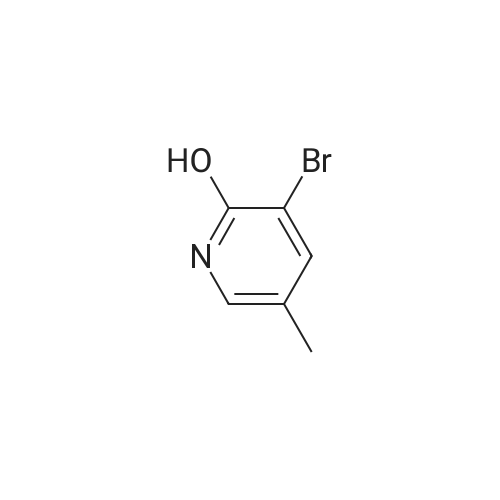
INTERMEDIATE 2; Step A; To a solution of 5-trifluoromethyl-2-pyridinol (51.0 g, 307 mmol) and sodium acetate (26.2 g, 319 mmol) in glacial acetic acid (200 mL) was added bromine (16.7 mL, 325 mmol) and the resulting mixture was heated at 80 C. for 2.5 hours. The reaction was allow to cool to room temperature and then was evaporated under reduced pressure. The residue was neutralized with saturated NaHCO3 solution and extracted with ethyl acetate (3×200 mL). The organic layers were combined, dried over MgSO4, filtered, and evaporated in vacuo to yield 74.45 g (98.7%) of the crude product. 1H NMR (400 MHz, CDCl3) delta 8.04 (d, J=2.6 Hz, 1H), 7.89 (m, 1H). |
| 98% |
With bromine; sodium acetate; In acetic acid; at 80℃; for 2.5h; |
To a solution of 5-trifluoromethyl-2-pyridinal (51 g, 310 mmol) and sodium acetate (26.2 g, 319 mmol) in glacial acetic acid (200 mL) was added bromine (16.7 mL, 325 mmol) and the resulting mixture was heated at 80 C. for 2.5 h. The reaction was allowed to cool to room temperature and then was evaporated under reduced pressure. The residue was neutralized with saturated NaHCO3 solution and extracted with ethyl acetate (3×200 mL). The organics were combined, dried over MgSO4, filtered, and evaporated in vacuo to yield 74.45 g (98%) of the crude product. 1H NMR (400 MHz, CDCl3) delta 8.04 (d, J=2.6 Hz, 1H), 7.89 (m, 1H). |
| 98.7% |
With bromine; sodium acetate; In acetic acid; at 80℃; for 2.5h; |
To a solution of 5-trifluoromethyl-2-pyridinol (51.0 g, 307 mmol) and sodium acetate (26.2 g, 319 mmol) in glacial acetic acid (200 mL) was added bromine (16.7 mL, 325 mmol) and the resulting mixture was heated at 80 C. for 2.5 hours. The reaction was allow to cool to room temperature and then was evaporated under reduced pressure. The residue was neutralized with saturated NaHCO3 solution and extracted with ethyl acetate (3×200 mL). The organic layers were combined, dried over MgSO4, filtered, and evaporated in vacuo to yield 74.45 g (98.7%) of the crude product. 1H NMR (400 MHz, CDCl3) delta 8.04 (d, J=2.6 Hz, 1H), 7.89 (m, 1H). |
| 95% |
With bromine; sodium acetate; In acetic acid; at 80℃; for 2h; |
To a solution of 5-TRIFLUOROMETHYL-2-PYRIDINOL (21.37 g, 131 mmol), and sodium acetate (11.23 g , 107 mmol) in glacial acetic acid was added bromine (6.94 ml, 135 mmol), and the resulting mixture stirred at 80 C for 2 hours. The cooled reaction mixture was evaporated and the residue basified by the addition of saturated NAHC03 (500 ml), and extracted with ethyl acetate (3 x 300 ml); the combined ethyl acetate layers were dried over MGS04, filtered and evaporated in vacuo to give the product (30.21 g, 95%) ; IH NMR 500MHZ (CDC13) 8 = 8.00 (1H, d, J = 2.29 Hz), 8.16 (LH, d, J=2. 29HZ). |
| 85% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 2h; |
lambda/-bromosuccinimide (NBS, 39.0Og, 0.22 mol) is added portionwise to a solution of 5-(trifluoromethyl)pyridin-2-ol (30.0Og, 0.18 mol) in DMF (180 ml_), and the resulting mixture is stirred for 2 hours. The mixture is poured into water (1200 mL) and the precipitate was collected by filtration. The crystal is dried in vacuo to give the product as a white solid (1st crystal : 28.1Og). The filtrate is extracted with EtOAc, and the organic layer is concentrated.The residue is poured into water and the precipitate is collected by filtration. The crystal is dried in vacuo to give 3-bromo-5-(trifluoromethyl)pyridin-2-ol (2nd crystal : 9.65 g, total:37.75g, 85 % yield) as a yellow solid.1H-NMR (400MHz, CDCI3), delta (ppm): 7.86 (d, 1H), 8.02 (d, 1H), 13.17 (br, 1H). |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 2h; |
V-bromosuccinimide (NBS, 39.0Og, 0.22 mol) is added portionwise to a solution of 5- {trifluoromethyl)pyridin-2-ol (30.0Og, 0.18 mol) in DMF (180 ml_), and the resulting mixture is stirred for 2 hours. The mixture is poured into water (1200 ml.) and the precipitate is collected by filtration. The crystal is dried in vacuo to give the product as a white solid (1st crystal : 28.1Og). The filtrate is extracted with EtOAc, and the organic layer is concentrated. The residue is poured into water and the precipitate is collected by filtration. The crystal is dried in vacuo to give 3-bromo-5-(trifluoromethyl)pyridin-2-ol as a yellow solid. 1H-NMR (400MHz, CDCI3), delta (ppm): 7.86 (d, 1 H), 8.02 (d, 1 H), 13.17 (br, 1 H). |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 2h; |
lambda/-bromosuccinimide (NBS, 39.0Og, 0.22 mol) is added portionwise to a solution of 5- (trifluoromethyl)pyridin-2-ol (30.0Og, 0.18 mol) in DMF (180 mL), and the resulting mixture is stirred for 2 hours. The mixture is poured into water (1200 mL) and the precipitate is collected by filtration. The crystal is dried in vacuo to give the product as a white solid (1st crystal : 28.1Og). The filtrate is extracted with EtOAc, and the organic layer is concentrated. The residue is poured into water and the precipitate is collected by filtration. The crystal is dried in vacuo to give 3-bromo-5-(trifluoromethyl)pyridin-2-ol as a yellow solid. <n="45"/>Case 505091H-NMR (400MHz, CDCI3), delta (ppm): 7.86 (d, 1 H), 8.02 (d, 1H), 13.17 (br, 1 H). |
|
With bromine; sodium acetate; In acetic acid; at 80℃; for 2.5h; |
INTERMEDIATE 4 To a solution of 5-trifluoromethyl-2-pyridinal (51 g, 310 mmol) and sodium acetate (26.2g, 319 mmol) in glacial acetic acid (200 mL) was added bromine (16.7 mL, 325 mmol) and the resulting mixture was heated at 80 C for 2.5 h. The reaction was allow to cool to room temperature and then was evaporated under reduced pressure. The residue was neutralized with saturated NaHC03 solution and extracted with ethyl acetate (3 x 200 mL). The organics were combined, dried over MgS04, filtered, and evaporated in vacuo to yield 74.45 g (98%) of the crude product. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping