| 36.4 g |
With toluene-4-sulfonic acid; In water; toluene;Reflux; |
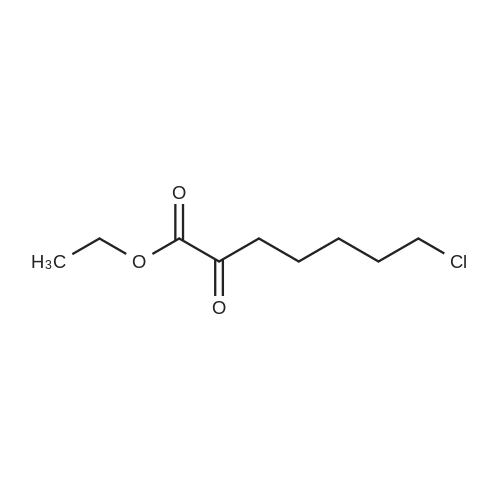
25.0 g of <strong>[78834-75-0]ethyl 7-chloro-2-oxoheptanoate</strong> obtained in Example 1 (GC normalized content 98.3%, external standard content 98.5%),(+) - S-2,2-dimethylcyclopropanecarboxamide and 0.15 g of p-toluenesulfonic acid were added to 125 ml of toluene, and the mixture was heated under reflux to azeotropize toluene with water to carry out the reaction.After completion of the reaction, the reaction mixture was cooled to room temperature, and the reaction solution was washed three times with 10% dilute hydrochloric acid (100 ml x 3) and 10% sodium hydrogen sulfite solution (100 ml x 3). After the washing, the toluene layer was separated and dried over anhydrous sodium sulfate. Filtered and concentrated under reduced pressure recovered toluene to give 36.4g of brown viscous liquid, the product will not be isolated directly used in the next step. |
|
With toluene-4-sulfonic acid; In toluene; at 130℃; for 10h; |
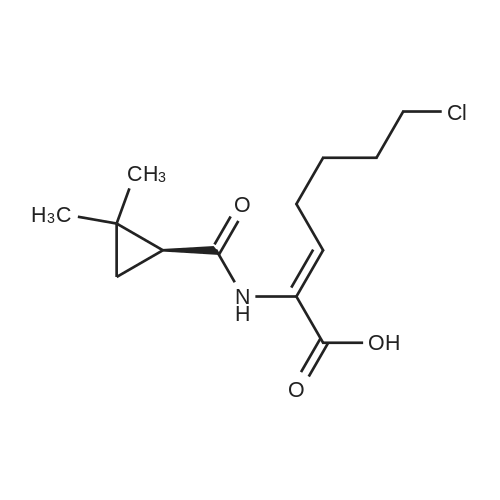
247.8 g of <strong>[78834-75-0]ethyl 7-chloro-2-oxoheptanoate</strong>,135.6 g of S - (+) 2,2-dimethylcyclopropylcarboxamidewith1.6 g of p-toluenesulfonic acid at 130 C1200ml toluene refluxed for 10 hours;After the reaction is completed, the toluene is concentrated and recovered;To the concentrate is added 600 ml of ethanol and 720 g of 10% sodium hydroxide solution,HPLC monitoring of the reaction process,At 45 ~ 50 for 10 hours;Then add t-butyl ether three times,Each 1000ml, discard the organic layer,Add concentrated hydrochloric acid,Adjust the pH to 3 ~ 3.5, add ethyl acetate three times,Each time 1000ml,Discard the water layer,Add anhydrous sodium sulfate drying,Concentration under reduced pressure gave (Z) -7-chloro-2- [(S) -2,2-dimethylcyclopropylcarboxamido] -2-heptenoic acid viscous liquid. Under reflux, 200 g of the above concentrate was added to 600 ml of dioxane,Then add 1260ml cyclohexane, stir,Let stand for 12 hours at room temperature, filtered,Drying in vacuo gave (Z) -7-chloro-2- [(S) -2,2-dimethylcyclopropylcarboxamido] -2-heptenoic acid as a solid; After controlling the temperature to be less than or equal to 10 DEG C, purging with nitrogen, 105.3 g of L-cysteine hydrochloride monohydrate was added, the mixture was stirred for 0.5 hours, warmed to 55-60 DEG C, the reaction was monitored by HPLC, the reaction was completed in 8 hours, Cooled to room temperature, washed three times with dichloromethane,Each 600ml, discard the organic layer,One volume of water was added to the reaction solution,Concentrated hydrochloric acid to adjust the pH to 2.5 to 3.0,Four times with dichloromethane,Each 800ml, discard the organic layer,That is included[R- [R *, S * (Z)]] - 7 - [(2-Amino-2- carboxyethyl) thio]2 - [[(2,2-dimethylcyclopropyl) carbonyl] amino] -2-heptenoic acid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping