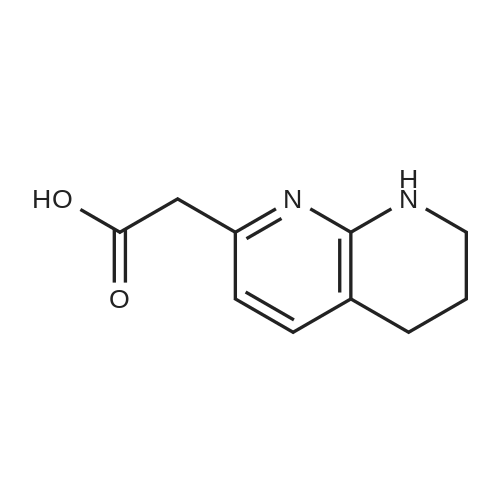
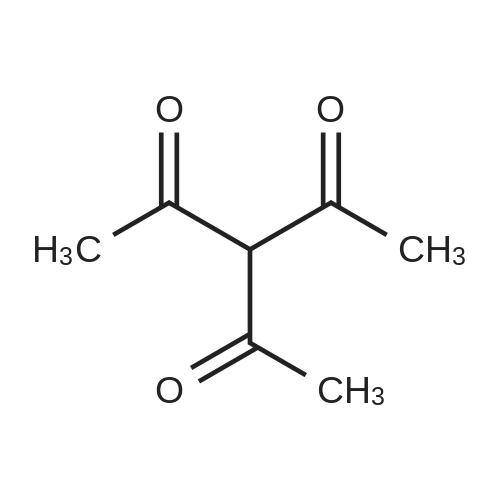
| 95% |
With L-proline; In ethanol; acetone; at 78 - 80℃; for 12h; |
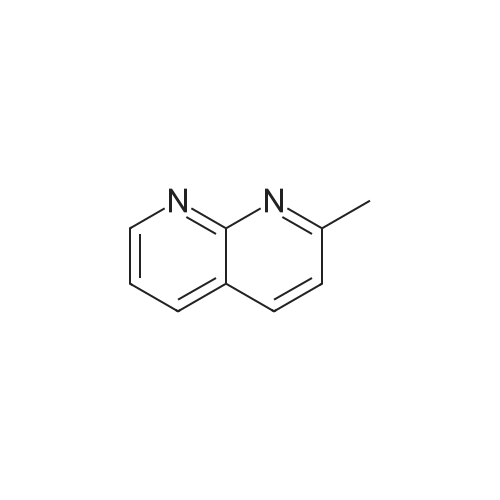
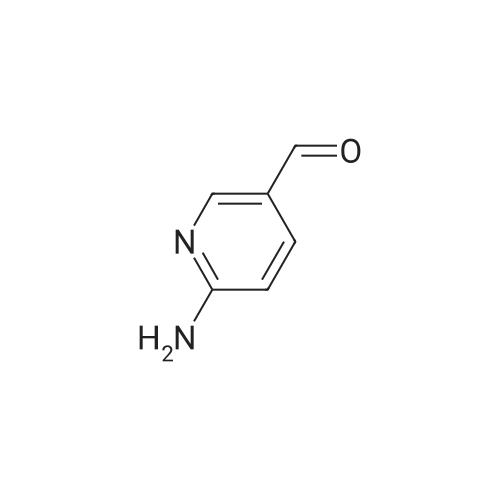
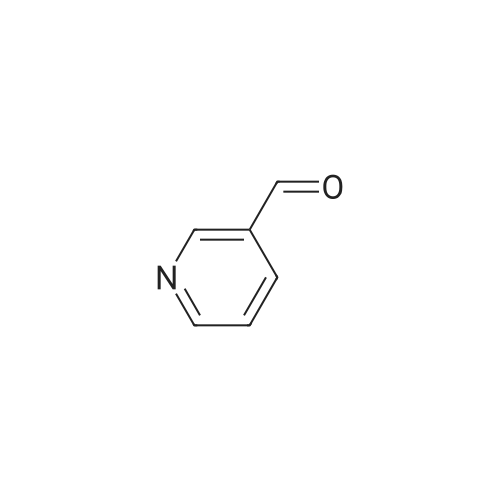
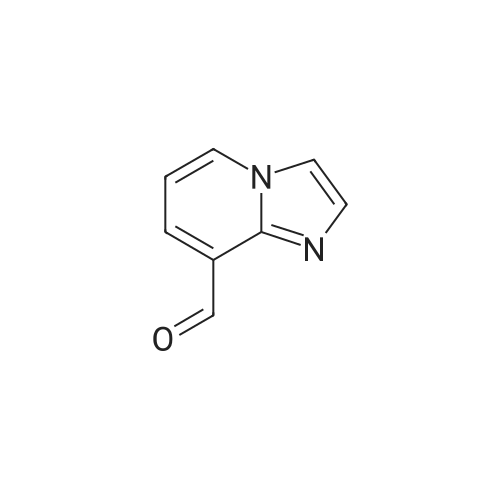
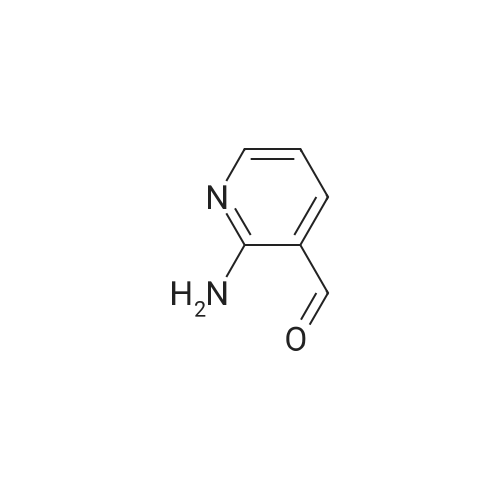
Step one: 2-amino-3-pyridinecarboxaldehyde (28mmol, 3.4g, purchased from Nanjing New Anjie Chemical Technology Co., Ltd. No. NE229) l-proline (28mmol, 3.2g, purchased from Beijing Coupling Technology Co., Ltd. 141218 The brand name) was dissolved in ethanol (100 mL), acetone (834 mmol, 48 g) was added, and the mixture was heated to reflux (78-80 C.) for 12 h. The solvent was distilled off under reduced pressure to obtain a crude solid product, which was then subjected to column chromatography (eluent). The volume ratio of 3/1 petroleum ether/ethyl acetate mixture was further purified to give 2-methyl-1,8-naphthyridine in a yield of 95%. |
| 90% |
With potassium hydroxide; In methanol; acetone;Reflux; |
Under a N2 atmosphere, a 100-mL flask was charged with 2-amino-3-pyridinecarboxaldehyde (2 g, 16.4 mmol) and dry acetone (20 mL). After the solution was refluxed for 5 min, a freshly prepared saturated solution of KOH in MeOH (0.1 mL) was added dropwise and the mixture was refluxed for 24 h. After cooling to room temperature, the solvent was removed under vacuum. The product was further purified by flash chromatography on a silica gel column to afford a brown yellow solid. m.p. 98-100 C. Yield: 90% (2.1 g, 14.8 mmol). 1H-NMR (400 MHz, CDCl3) delta (ppm): 9.08 (dd, J = 4.0 Hz, J = 1.6 Hz, 1H), 8.16 (dd, J = 8.0 Hz, J = 2.0 Hz, 1H), 8.09 (d, J = 8.0 Hz, 1H), 7.45 (dd, J = 8.0 Hz, J = 4.4 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 2.83 (s, 1H); 13C-NMR (100 MHz, CDCl3) delta: 162.9, 155.8, 153.2, 136.7, 122.9, 121.2, 120.6, 25.5 ppm. Anal. Calcd for C9H8N2: C 74.98; H 5.59, N 19.43. Found: C 74.88; H 5.47, N 19.65. |
| 88.3% |
With methanol; potassium hydroxide; at 55℃; for 6h; |
To a stirred solution of 2-amino-3-formyl pyridine (7 g, 57 mmol) in acetone (70 mL), a saturated solution of KOH in methanol (0.5 mL) was added and the mixture was stirred at 55 C for 6 h. Completion of the reaction was monitored by TLC. The mixture was concentrated and the resulting crude product was purified by flash chromatography (Elutant: 65-85 % EtOAc in pet ether) to give the title compound. Yield: 88.3% (7.3 g, palebrown solid). 1H NMR (400 MHz, CDCI3): 69.10-9.09 (m, IH), 8.18-8.15 (m, IH), 8.09 (d, J = 8.4 Hz, IH), 7.47-7.39 (m, IH), 7.29-7.28 (m, IH), 2.84 (s, 3H). LCMS: (Method B) 145.0 (M +H), Rt. 3.06 mm, 97.85% (Max). HPLC: (Method B) Rt 2.98 mm, 98.09% (Max). |
| 88.3% |
With potassium hydroxide; In methanol; acetone; at 55℃; for 6h; |
To a stirred solution of 2-amino-3-formyl pyridine (7 g, 57 mmol) in acetone (70 mL), a saturated solution of KOH in methanol (0.5 mL) was added and the mixture was stirred at 55 C for 6 h. Completion of the reaction was monitored by TLC. The mixture was concentrated and the resulting crude product was purified by flash chromatography (Elutant: 65-85 % EtOAc in pet ether) to give the title compound. Yield: 88.3% (7.3 g, pale brown solid). 1H NMR (400 MHz, CDCI3): delta 9.10-9.09 (m, 1 H), 8.18-8.15 (m, 1 H), 8.09 (d, J = 8.4 Hz, 1 H), 7.47-7.39 (m, 1 H), 7.29-7.28 (m, 1 H), 2.84 (s, 3H). LCMS: (Method B) 145.0 (M +H), Rt. 3.06 min, 97.85% (Max). HPLC: (Method B) Rt 2.98 min, 98.09% (Max). |
| 69% |
With piperidine; In ethanol; for 24h;Heating / reflux; |
The compound was prepared according to the procedure as described by E. M. Hawes and D. G. Wibberley, J. Chem. Soc. (C), 1966, 315. To a solution of 2-amino-3-pyridinecarboxaldehyde (2 g, 16 mmol) in ethanol 3 ml) was added acetone (1.9 g, 32 mmol) and peperidine (0,34 g, 4 mmol) and the reaction mixture was refluxed 24 hours. Reaction mixture was cooled to room temperature then concentrated in vacuum. Ether was added to concentrated residue. Solid was filtered and dried to give 1.62 g (69%) yellow solid. NMR (CD3OD) delta 2.76 (s, 3H), 7.52-7.58 (m, 2H), 8.30 (d, 2H, J=8.33 Hz), 8.36-8.39 (m, 1H), 8.39-8.99 (m, 1H). |
| 69% |
With piperidine; In ethanol; for 24h;Heating / reflux; |
To a solution of 2-amino-3-pyridinecarboxaldehyde (2 g, 16 mmol) in ethanol3 mL) was added acetone (1.9 g, 32 mmol) and piperidine (0.34 g, 4 mmol and the reaction10 mixture was refluxed 24 hours. Reaction mixture was cooled to room temperature thenconcentrated in vacuum. Ether was added to concentrated residue. Solid was filtered anddried to give 1.62 g (69%) yellow solid. NMR (CD3OD) 6 8.39-8.99 (m, 1H), 8.36-8.39 (m,1H), 8.30 (d, 2H, J = 8.33 Hz), 7.52-7.58 (m, 2H), 2.76 (s, 3H). M + H - 145. |
|
With L-proline; In ethanol; for 8h;Reflux; |
(e) 2-Methyl-l ,8-naphthyridine[00448] To a suspension of 2-aminonicotinaldehyde (732 mg, 6 mmol) and L-proline (69 mg, 0.6 mmol) in EtOH (15 mL) was added acetone (1.74 g, 30 mmol). Then the mixture was heated at reflux and stirred for 8 h. The resulting mixture was concentrated under reduced pressure to give a residue, which was washed with water (10 mL) and extracted with DCM (15 mL x 3). The combined organic layers were washed with brine (10 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford the crude product 2-methyl-l,8-naphthyridine as a yellow solid (768 mg). MS (ESI): m/z 145 [M+H]+. See e.g. , Bioorg. Med. Chem. Lett., 2005, 15, 2679-84. |
|
With L-proline; In ethanol; |
Int-19A was prepared using the procedure described in Patent: WO 2011150156. NMR (500MHz, DMSO-c) delta 9.02 (dd, J = 4.3, 2.1 Hz, 1H), 8.40 (dd, J = 8.0, 1.9 Hz, 1H), 8.34 (d, J = 8.3 Hz, 1H), 7.56 (dd, J = 8.1, 4.3 Hz, 1H), 7.51 (s, 1H), 2.70 (s, 3H). HPLC retention time (Method 1): 0.303 mia; LCMS (ES): m/z 145.0 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping