|
With triphenylphosphine; diethylazodicarboxylate; In dichloromethane; at 25℃; for 1h; |
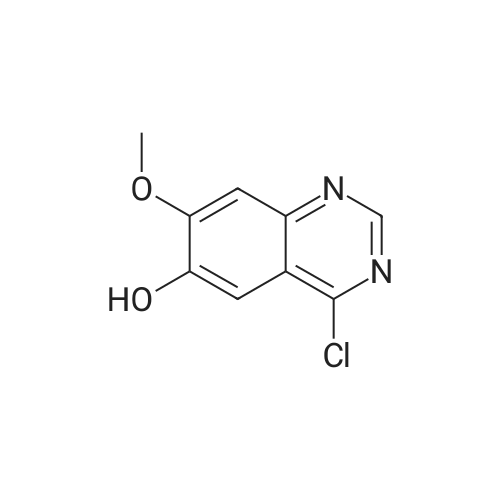
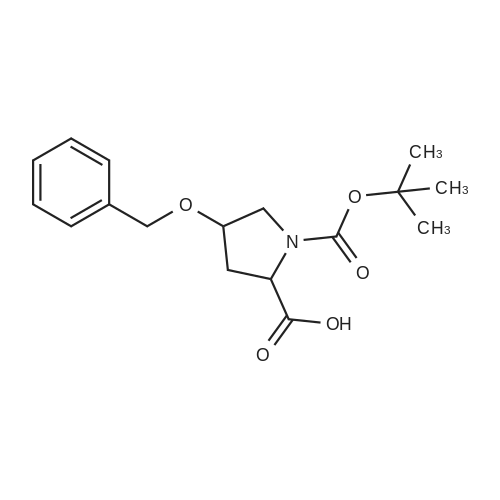
Example 12 (4R)-4-({4-[(3-chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl}oxy)-1-methyl-N- PROP-2-VN-1-VL-L-PROLINAMIDE Example 12 HATU (0.34g) was added to an agitated solution of (4R)-4-({4-[(3-CHLORO-2- fluorophenyl) amino]-7-methoxyquinazolin-6-yl} oxy)-1-methyl-L-proline (0.2g), propargylamine (49.3mg) and DIPEA (231mg) in DIMETHYLACETAMIDE (10ML). THE MIXTURE was stirred at 50C for 10 minutes then allowed to stand at room temperature overnight. The reaction mixture was reduced in vacuo. The residues were re-dissolved in methylene chloride and washed with sodium hydroxide solution (2M) and water. The organic phase was then purified by column chromatography on silica eluting with increasingly polar mixtures of METHANOL/METHYLENE chloride (0/100-12/88). The fractions containing the desired product were combined and evaporated to a foam which was triturated with diethylether to give the title compound as a white solid. (0. 067G). LH NMR Spectrum (DMSO D6) 2.08-2. 22 (M, 1H), 2.25-2. 62 (M, 2H + DMSO), 2.31 (s, 3H), 3.06 (s, 1H), 3.15 (t, 1H), 3.62-3. 72 (M, 1H), 3.78-4. 02 (M, 2H), 3.93 (s, 3H), 5.06 (M, 1H), 7.16-7. 32 (M, 1H), 7.21 (s, 1H), 7.43- 7.56 (M, 2H), 7.67 (s, 1H), 8.28 (M, 1H), 8.36 (s, 1H), 9.63 (s, 1H); Mass Spectrum : (M+H) + 484. The starting material was prepared as follows: 1-TERT-BUTYL 2-methyl (2S, 4S)-4-HYDROXYPYRROLIDINE-1, 2-dicarboxylate (1), (Boc-cis- Hyp-OMe) is commercially available. Di-ethyl azodicarboxylate (12.4g) was added slowly to a stirred suspension of 1-TEST- butyl 2-methyl (2S, 4S)-4-HYDROXYPYRROLIDINE-1, 2-dicarboxylate (1) (17.46g), 4-chloro-7- methoxyquinazolin-6-ol (2) (10g) [prepared as described in Example 1 above (compound 3)] and TRIPHENYLPHOSPHINE (L8. 67G) INMETHYLENE chloride (300 ML) at 25C under an atmosphere of nitrogen and the reaction mixture was stirred for 1 hours. The reaction mixture was then evaporated to ? volume and purified by column chromatography on silica eluting with increasingly polar mixtures of METHANOL/METHYLENE chloride (0/100-3.5/96. 5). The desired product fractions were combined and evaporated to give 1-TERT-BUTYL 2-methyl (2S, 4R)-4- [ (4- CHLORO-7-METHOXYQUINAZOLIN-6-YL) oxy] pyrrolidine-1, 2-dicarboxylate (3) as a pale yellow foam Mass Spectrum : (M+H) + 438. This was used in the preparation of (4) without further purification. The starting material (4) was prepared as follows: 4. 0M HCl in Dioxane (39.2 ML) was added to a suspension of 1-tert-butyl 2-methyl (2S, 4R)-4- [ (4-chloro-7-methoxyquinazolin-6-yl) oxy] pyrrolidine-1, 2-dicarboxylate (3) and 3- CHLORO-2-FLUOROANILINE (7.61g) in acetonitrile (300 ML) and the reaction mixture was stirred and heated at 50C for 1 hours. The resulting precipitate was filtered hot and washed with acetonitrile and diethylether and dried under vacuum to give methyl (4R)-4-({4-[(3-CHLORO-2- fluorophenyl) AMINO]-7-METHOXYQUINAZOLIN-6-YL} OXY)-L-PROLINATE HYDROCHLORIDE (4) as an off- white solid, (23. 05G). 1H NMR Spectrum : (DMSO D6) 2.46-2. 74 (M, 2H), 3.24-3. 68 (m, 1H), 3.78 (s, 3H), 3.95-4. 07 (M, 1H), 4.00 (s, 3H), 4.61 (t, 1H), 5.50 (M, 1H), 7.35 (t, 1H), 7.47-7. 57 (M, 1H), 7.49 (s, 1H), 7.63 (t, 1H), 8.73 (s, 1H), 8.83 (s, 1H), 12.38 (bs, LH)-, MASS SPECTRUM : (M+H) + 447. Methyl (4R)-4-({4-[(3-CLZLORO-2-FLUOROPHENYL) AMINO]-7-METHOXYQUINAZOLIN-6-YL} OXY)- L-PROLINATE HYDROCHLORIDE (4) (22.9g), paraformaldehyde (14.25g), sodium cyanoborohydride (11.97g) and magnesium sulphate (11.4g) were suspended in methanol (600ML) and heated at 45C for 3 hours under an atmosphere of nitrogen. The reaction mixture was filtered, evaporated and partitioned between ethylacetate and saturated aqueous sodium bicarbonate solution. The organics were then washed with saturated brine, dried over MgS04, filtered and evaporated. The residues were then purified by column chromatography on silica eluting with increasingly polar mixtures of methanol/methylene chloride (0/100-10/90) to give methyl (4R)- 4-({4-[(3-CHLORO-2-FLUOROPHENYL) AMINO] -7-METHOXYQUINAZOLIN-6-YL} OXY)-L-METHYL-L-PROLINATE (5) as a yellow solid, (14. 87 G). LH NMR SPECTRUM : (DMSO d6) 2.13-2. 25 (M, 1H), 2.34 (s, 3H), 2.46-2. 61 (M, 2H + DMSO), 3.37 (t, 1H), 3.57-3. 69 (M, 1H), 3.66 (s, 3H), 3.93 (s, 3H), 5.08 (M, 1H), 7.21 (s, 1H), 7.23-7. 31 (t, 1H), 7.43-7. 58 (M, 2H), 7.69 (s, 1H), 8.37 (s, 1H), 9.62 (s, 1H) ; Mass Spectrum : (M+H) + 461. Sodium hydroxide 2M (24.2 ml) was added to a stirred solution of methyl (4R)-4- ( {4- [(3-CHLORO-2-FLUOROPHENYL) AMINO]-7-METHOXYQUINAZOLIN-6-YL} OXY)-1-METHYL-L-PROLINATE (5) (14.87g) in methanol (100 ml) at 25C and the reaction mixture was stirred for 1 hour. The reaction mixture was evaporated and the residue re-dissolved in water. The pH of this solution was then adjusted to 6 by the dropwise addition of 2M HCl (aq) to give (4R0-4-({4-[(3-chloro- 2-fluorophenyl) AMINO]-7-METHOXYQUINAZOLIN-6-YL} OXY)-L-METHYL-L-PROLINE (6) as a pale yellow solid which was filtered and washed with water and dried, (1... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping