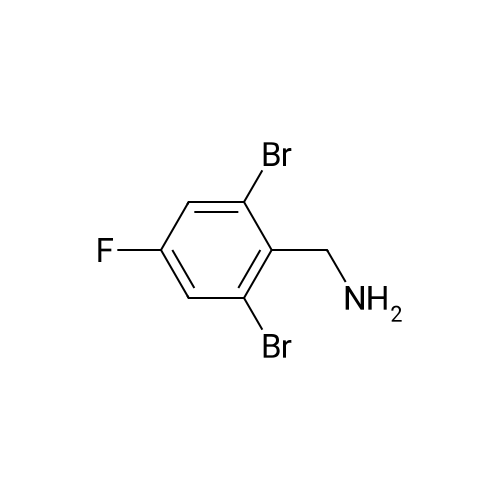
Alternatived Products of [ 747392-34-3 ]
Product Details of [ 747392-34-3 ]
| CAS No. : | 747392-34-3 |
MDL No. : | MFCD06212850 |
| Formula : |
C7H7BrFN
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | HEVQVBQUMCXTJO-UHFFFAOYSA-N |
| M.W : |
204.04
|
Pubchem ID : | 2773357 |
| Synonyms : |
|
Safety of [ 747392-34-3 ]
Application In Synthesis of [ 747392-34-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 747392-34-3 ]
- 1
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
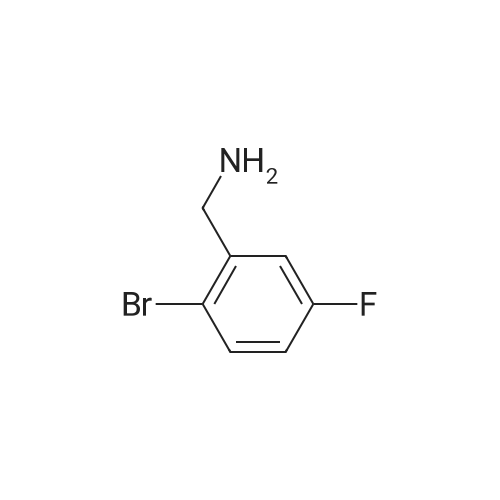
 [ 10439-23-3 ]
[ 10439-23-3 ]

-
 [ 880104-97-2 ]
[ 880104-97-2 ]
- 2
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
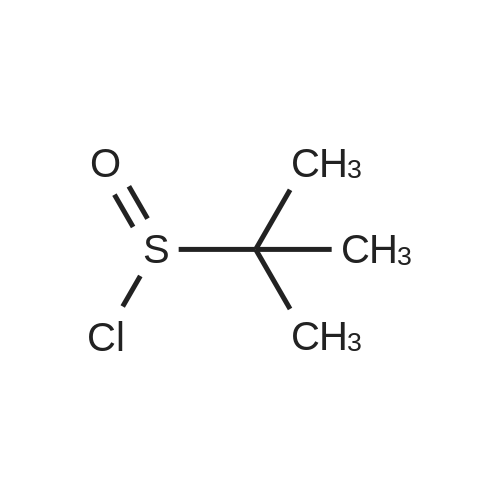 [ 31562-43-3 ]
[ 31562-43-3 ]

-
2-methyl-propane-2-sulfinic acid 2-bromo-5-fluoro-benzylamide
[ No CAS ]
- 3
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
5-fluoro-2,3-dihydro-benzo[<i>d</i>]isothiazole 1-oxide
[ No CAS ]
- 4
-
 [ 1198105-01-9 ]
[ 1198105-01-9 ]

-
 [ 747392-34-3 ]
[ 747392-34-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 46% |
With potassium hydroxide; triphenylphosphine; In tetrahydrofuran; water; |
2-Bromo-5-fluorobenzyl bromide (3.0 g, 11 mmol, 1.0 equiv) was dissolved in dimethyl sulfoxide (100 mL) at ambient temperature. Sodium azide (2.8 g, 44 mmol, 4.0 equiv) was added to the solution and the reaction mixture heated at reflux for one day. The solution was cooled, quenched with water, and then extracted with ethyl acetate. The organic extracts were combined, washed with brine, dried over sodium sulfate and concentrated in vacuo. Purification by column chromatography (10: 1 hexanes/ethyl acetate) afforded the azide (0.85 g, [34%).] The azide (0.85 g, 3.7 mmol, 1.0 equiv) was dissolved in tetrahydrofuran (25 mL) and water (5.0 mL). KOH (0.20 g, 3.6 mmol, 0.97 equiv) was added to the solution followed by triphenylphosphine (1.1 g, 4.4 mmol, 1.2 equiv). The reaction mixture was stirred overnight at ambient temperature. The reaction was quenched with hydrochloric acid (conc.) and extracted with ethyl acetate. The aqueous layers were combined and made basic with sodium hydroxide (pellet) until pH [REACHED-14.] The aqueous layer was extracted with ethyl acetate, the organic layers combined, washed with brine, dried over sodium sulfate and concentrated in vacuo to afford the crude amine (0.35g, [46%)] as an orange oil. |
- 5
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
 [ 629628-16-6 ]
[ 629628-16-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
2-Bromo-5-fluorobenzyl amine [(0. 35 G,] 1.7 mmol, 1.0 equiv) was dissolved in tetrahydrofuran (20 mL) and stirred overnight at ambient temperature. The solvent was removed in vacuo and water (30 mL) was used to dissolve the concentrate. The solution was heated at reflux overnight and then cooled to ambient temperature. Sodium hydroxide (pellet) was added to the solution until the pH was-14. The solution was extracted with ethyl acetate, the organic extracts combined, washed with brine and dried over sodium sulfate. The solution was concentrated in vacuo. The concentrate was purified by column chromatography (30: 1 chloroform/methanol sat'd with ammonia). The product was isolated as a white solid. H NMR (300 MHz, [CDC13] w/TMS): [57.] 46-7.50 (m, 1H), 7.16-7. 19 (m, 1H), 6.83-6. 90 (m, 1H), 4.43 (s, 2H), 4.31 (t, 2H, J= 8.5 Hz), 3.79 (t, 2H, J= 8.8 Hz). |
- 6
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
 [ 31562-43-3 ]
[ 31562-43-3 ]

-
2-methyl-propane-2-sulfinic acid 2-bromo-5-methoxy-benzylamide
[ No CAS ]
- 7
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
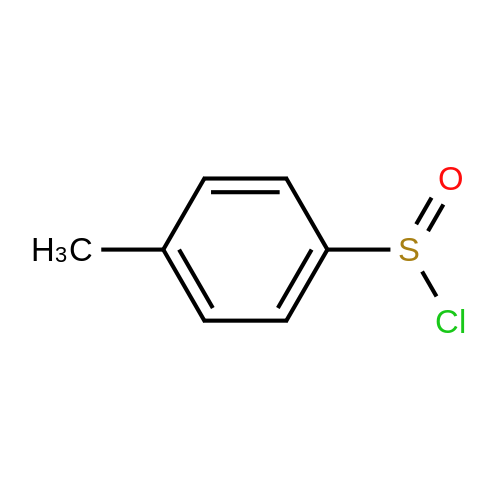
 [ 4214-28-2 ]
[ 4214-28-2 ]

-
 [ 1257871-98-9 ]
[ 1257871-98-9 ]
- 8
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
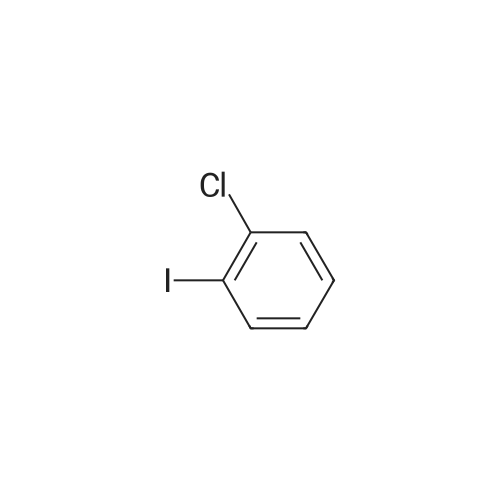 [ 615-41-8 ]
[ 615-41-8 ]

-
 [ 1257871-99-0 ]
[ 1257871-99-0 ]
- 9
-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
 [ 1169762-17-7 ]
[ 1169762-17-7 ]

-
 [ 1169762-19-9 ]
[ 1169762-19-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetonitrile; at 80℃; for 10h;Inert atmosphere; |
General procedure: To a solution of tert-butyl 2-cyano-3,3-bis(methylthio) acrylate (4) (245 mg; 1.0 mmol) in CH3CN (3.0 ml) was added R1R2NH (1.0 mmol), and the mixture was stirred at 50 C for 1 h. DBU (0.3 ml; 2.0 mmol) and R3-PhCH2NH2 (1.2 mmol) were then added to the mixture, and the new mixture was stirred for 10 h at 80 C. The mixture was next diluted with EtOAc, washed with H2O, 1 N HCl aq, satd NaHCO3 aq and brine, and dried over Na2SO4, and the filtrate was concentrated in vacuo. To the residue in DMF (3.0 ml) was added K2CO3 (415 mg; 3.0 mmol) and then ethyl bromoacetate (0.13 ml; 1.3 mmol) dropwise, and the reaction mixture was stirred for 2 h at 50 C. The resulting slurry was diluted with EtOAc, washed with satd NH4Cl aq and brine and dried over Na2SO4, and the filtrate was concentrated in vacuo. To tert-butanol (1.5 ml) was added LiNH2 (58 mg; 2.5 mmol) and the mixture was stirred for 10 min at 80 C and then cooled to room temperature, and CH3CN (2.0 ml) was added. To the prepared slurry was added dropwise the alkylated intermediate in toluene (1.0 ml), and the resulting mixture was stirred for 2 h at 30 C. The slurry was diluted with EtOAc, washed with satd NH4Cl aq, and brine and dried over Na2SO4, and the filtrate was concentrated in vacuo. Finally, the residue was purified by column chromatography (hexane/EtOAc=8/1 to 3/1). |
- 10
-
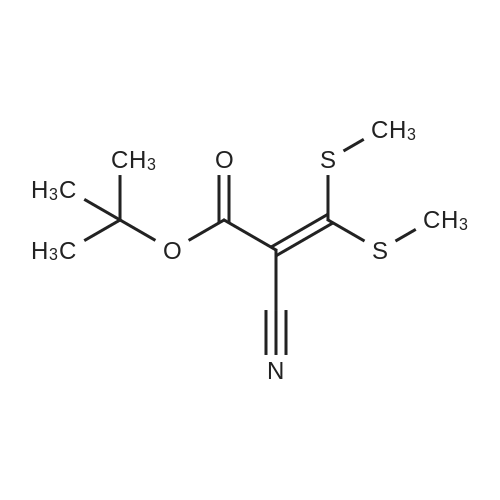 [ 913543-99-4 ]
[ 913543-99-4 ]

-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
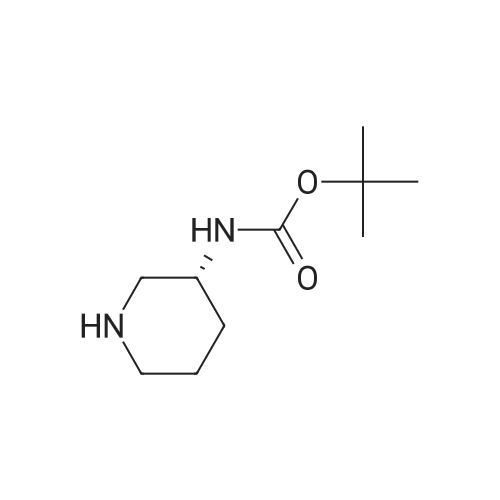 [ 309956-78-3 ]
[ 309956-78-3 ]

-
C25H34BrFN4O4
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
General procedure: A mixture of 14 (5.00 g; 20.4 mmol) and 13a (4.37 g; 20.4 mmol) in CH3CN (60 ml) was stirred at 50 C for 1 h. After cooling to room temperature, 1,8-diazabicyclo[5,4,0]undeca-7-ene (DBU) (6.20 g; 40 mmol) and 2-bromo-5-fluoro-benzylamine (4.99 g; 24.4 mmol) were added to the mixture, and the resulting mixture was stirred at 80 C for 10 h. After removal of the solvent, the residue was diluted with EtOAc, and washed with H2O, 10%KHSO4 aq, 10%NaOH aq and brine. The EtOAc layer was dried over Na2SO4 and the filtrate was concentrated. The residue was dissolved in DMF (60 mL), and ethyl bromoacetate (2.80 mL; 28 mmol) and K2CO3 (8.5 g) were added. The resulting slurry was then stirred at 50 C for 2 h and cooled to room temperature. The slurry was diluted with EtOAc, filtered through Celite, and washed with satd NH4Cl aq and brine. The organic layer was dried over Na2SO4, and the filtrate was concentrated. To tert-butyl alcohol (30 mL) was added lithium amide (1.16 g; 48 mmol). After stirring at 80 C for 1 h, the resulting mixture was cooled to 30 C, and CH3CN (40 mL) was added. A solution of the alkylated product from the third step in toluene (10 mL) was next added dropwise, and the reaction mixture was stirred for 2 h at 30 C. The solvent was removed, and to the residue was added EtOAc. The resulting mixture was finally washed with satd NH4Cl aq and brine, the organic layer was dried over Na2SO4, and the filtrate was concentrated. The residue was purified by SiO2 column chromatography (Hexane/EtOAc = 6/1 to 3/1) to give 5.80 g (44% from 14) of 15a as amorphous solid. In addition to the target product 15a, the ester exchange product (R2 = tert-butyl) was also obtained as an amorphous (415 mg). |
- 11
-
 [ 913543-99-4 ]
[ 913543-99-4 ]

-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
tert-butyl [3-methylpiperidin-3-yl]carbamate
[ No CAS ]

-
C26H36BrFN4O4
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
A mixture of 14 (5.00 g; 20.4 mmol) and 13a (4.37 g; 20.4 mmol) in CH3CN (60 ml) was stirred at 50 C for 1 h. After cooling to room temperature, 1,8-diazabicyclo[5,4,0]undeca-7-ene (DBU) (6.20 g; 40 mmol) and 2-bromo-5-fluoro-benzylamine (4.99 g; 24.4 mmol) were added to the mixture, and the resulting mixture was stirred at 80 C for 10 h. After removal of the solvent, the residue was diluted with EtOAc, and washed with H2O, 10%KHSO4 aq, 10%NaOH aq and brine. The EtOAc layer was dried over Na2SO4 and the filtrate was concentrated. The residue was dissolved in DMF (60 mL), and ethyl bromoacetate (2.80 mL; 28 mmol) and K2CO3 (8.5 g) were added. The resulting slurry was then stirred at 50 C for 2 h and cooled to room temperature. The slurry was diluted with EtOAc, filtered through Celite, and washed with satd NH4Cl aq and brine. The organic layer was dried over Na2SO4, and the filtrate was concentrated. To tert-butyl alcohol (30 mL) was added lithium amide (1.16 g; 48 mmol). After stirring at 80 C for 1 h, the resulting mixture was cooled to 30 C, and CH3CN (40 mL) was added. A solution of the alkylated product from the third step in toluene (10 mL) was next added dropwise, and the reaction mixture was stirred for 2 h at 30 C. The solvent was removed, and to the residue was added EtOAc. The resulting mixture was finally washed with satd NH4Cl aq and brine, the organic layer was dried over Na2SO4, and the filtrate was concentrated. The residue was purified by SiO2 column chromatography (Hexane/EtOAc = 6/1 to 3/1) to give 5.80 g (44% from 14) of 15a as amorphous solid. In addition to the target product 15a, the ester exchange product (R2 = tert-butyl) was also obtained as an amorphous (415 mg). |
- 12
-
 [ 913543-99-4 ]
[ 913543-99-4 ]

-
 [ 747392-34-3 ]
[ 747392-34-3 ]

-
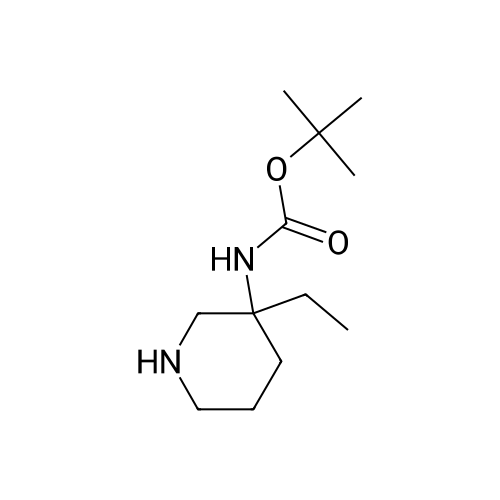 [ 1169762-29-1 ]
[ 1169762-29-1 ]

-
C27H38BrFN4O4
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
General procedure: A mixture of 14 (5.00 g; 20.4 mmol) and 13a (4.37 g; 20.4 mmol) in CH3CN (60 ml) was stirred at 50 C for 1 h. After cooling to room temperature, 1,8-diazabicyclo[5,4,0]undeca-7-ene (DBU) (6.20 g; 40 mmol) and 2-bromo-5-fluoro-benzylamine (4.99 g; 24.4 mmol) were added to the mixture, and the resulting mixture was stirred at 80 C for 10 h. After removal of the solvent, the residue was diluted with EtOAc, and washed with H2O, 10%KHSO4 aq, 10%NaOH aq and brine. The EtOAc layer was dried over Na2SO4 and the filtrate was concentrated. The residue was dissolved in DMF (60 mL), and ethyl bromoacetate (2.80 mL; 28 mmol) and K2CO3 (8.5 g) were added. The resulting slurry was then stirred at 50 C for 2 h and cooled to room temperature. The slurry was diluted with EtOAc, filtered through Celite, and washed with satd NH4Cl aq and brine. The organic layer was dried over Na2SO4, and the filtrate was concentrated. To tert-butyl alcohol (30 mL) was added lithium amide (1.16 g; 48 mmol). After stirring at 80 C for 1 h, the resulting mixture was cooled to 30 C, and CH3CN (40 mL) was added. A solution of the alkylated product from the third step in toluene (10 mL) was next added dropwise, and the reaction mixture was stirred for 2 h at 30 C. The solvent was removed, and to the residue was added EtOAc. The resulting mixture was finally washed with satd NH4Cl aq and brine, the organic layer was dried over Na2SO4, and the filtrate was concentrated. The residue was purified by SiO2 column chromatography (Hexane/EtOAc = 6/1 to 3/1) to give 5.80 g (44% from 14) of 15a as amorphous solid. In addition to the target product 15a, the ester exchange product (R2 = tert-butyl) was also obtained as an amorphous (415 mg). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping