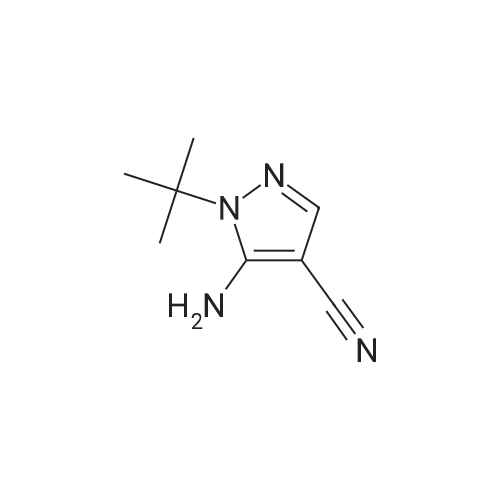
| 83% |
With triethylamine; In ethanol; for 3.0h;Reflux; |
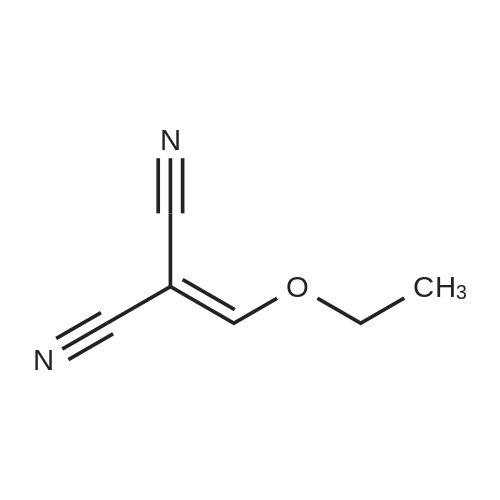
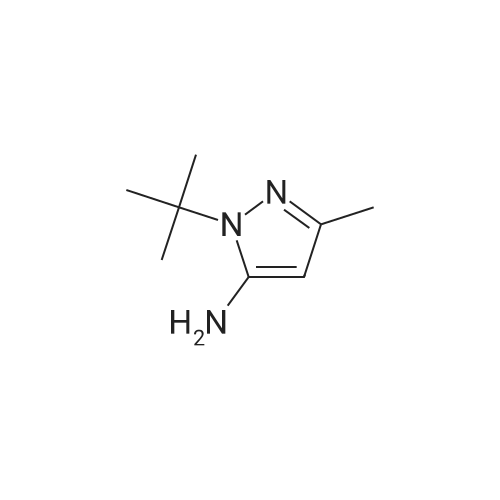
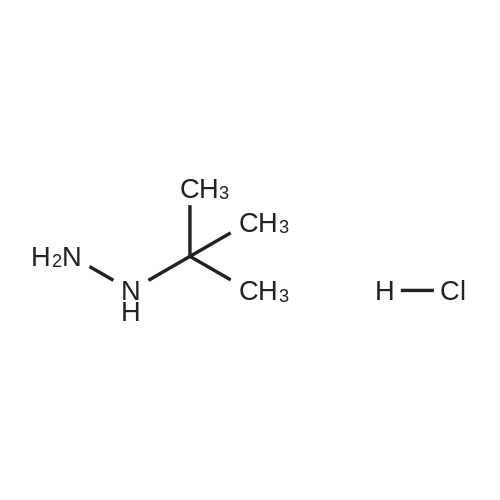
In tert-butylhydrazine hydrochloride (8.67 g, 69.6 mmol)Was added triethylamine (9.7 mL, 69.6 mmol)After adding anhydrous ethanol (460 mL), the mixture was stirred and dissolved at room temperature,Ethoxymethylenemalononitrile (8.5 g, 69.6 mmol) was added in small portions.After heating the solution to reflux for 3 hours,After cooling, the solvent was evaporated to give an orange solid.And extracted with ethyl acetate (0.5 L) and water (0.25 L)After drying by adding magnesium sulfate,The organic layer was evaporated to give an orange-yellow solid.The resulting solid was continuously washed with a 10% ethyl acetate in cyclohexane solution to give a crystalline solid5-amino-1-tert-butyl hydrogen - pyrazol-4-cyano 9.54g(Yield: 83%). |
| 83% |
With triethylamine; In ethanol; at 20.0℃; for 3.0h;Reflux; |
Triethylamine (9.7mL, 69.6mmol) was added to tert-butylhydrazine hydrochloride (8.67g, 69.6mmol) After adding absolute ethanol (460mL) and stirring at room temperature to dissolve, Ethoxymethylenemalononitrile (8.5 g, 69.6 mmol) was added in small portions, the solution was heated to reflux for 3 hours, and then the solvent was evaporated in vacuo to obtain a crude orange product. It was extracted with a mixed solution of ethyl acetate (0.5 L) and water (0.25 L), dried over anhydrous magnesium sulfate, and the organic layer was evaporated to obtain an orange solid. Continue to wash the obtained solid with a cyclohexane solution containing 10% ethyl acetate to obtain crystalline solid 5-amino-1-tert-butyl 1hydro-pyrazole-4-cyano 9.54g (yield 83% ). |
| 64.4% |
With triethylamine; In ethanol; for 3.0h;Heating / reflux; |
A mixture of t-butylhydrazine hydrochloride (4.67 g, 53 mmol) and triethylamine (5.35 g, 53 mmol) in anhydrous ethanol (250 ml) was stirred and ethoxymethylene malononitrile (6.47 g, 53 mmol) was slowly added in portions. The mixture was heated at reflux for 3 hr. The solvent was removed in vacuo and the product was crystallized from ethyl acetate -hexane followed by ether to afford the title compound as light pale brown crystals (5.6 g, 64.4 %); LC/MS, API-ES, Neg, (M-H)", 163.0. |
|
With triethylamine; In ethanol; for 3.0h;Heating / reflux; |
A mixture of t-butylhydrazine hydrochloride (4.67 g, 53 mmol) and triethylamine (5.35 g, 53 mmol) in anhydrous ethanol (250 ml) was stirred and ethoxymethylene malononitrile (6.47 g, 53 mmol) was slowly added in portions. The mixture was heated at reflux for 3 hr. The solvent was removed in vacuo and the product was crystallized from ethyl acetate - hexane followed by ether to afford 5-amino-l-tert-butyl-lH-pyrazole-4-carbonitrile as light pale brown crystals (5.6 g, 34.1 mmol); LC/MS, API-ES, Neg, (M-H)", 163.0. |
|
With triethylamine; In ethanol; at 82.0℃; for 3.0h;Inert atmosphere; |
[0814] Example 1: 5-amino-1-(tert-butyl)-1H-pyrazole-4-carbonitrile (I1): . In a 250mL flame-dried argon purged round bottom flask, triethylamine (1.78g, 17.7mmol), and t- butyl hydrazine hydrochloride (1.56g, 12.5mmol) are dissolved in anhydrous ethanol (85mL). Ethoxymethylenemalononitrile (1.98g, 17.7mmol) is added slowly and reaction mixture is brought to reflux at 82C for 3 hours. The solvent is removed in vacuo and 10% ethyl acetate / hexane is added (5mL) and the mixture is sonicated (or simply utilize recrystallization from 10% ethyl acetate / hexane). The resulting crystalline solid is filtered, and washed with ether to yield I1. LC-MS (ES+) calcd for C8H12N4 (M+H)+ 165.11, found 165.05. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping