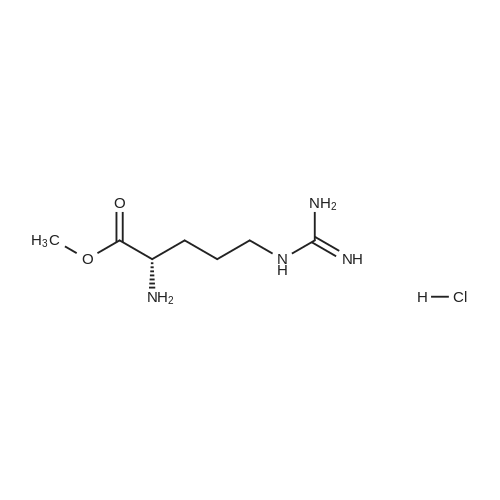
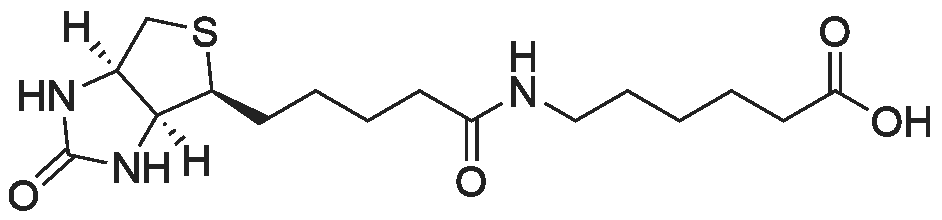
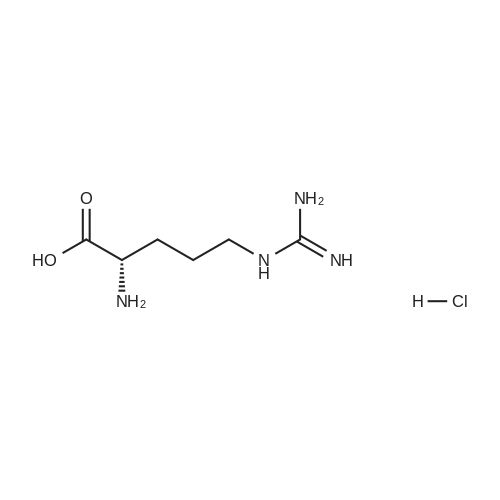
|
With hydrogenchloride; In 1,4-dioxane; |
General procedure: TLC plates were purchased from Merck KGaA, Millipore Corporation. Flash Column Chromatography performed using silica gel from Sorbent Technologies in Chemglass fritted columns. Silica Dimensions: porosity 60 A, particle size: 40-63 mm, 230 x 400 mesh. Preparative HPLC performed on a Gilson PLC 2020. Column: Synergi 4m Fusion-RP by Phenomenex, 150 x 21.2 mm. Elution performed with a step gradient of 5% CH3CN in H2O for 5 min followed by a ramp to 1:1 CH3CN/H2O over 12 min (no buffers were used) at 20 mL/min flow rate. Compounds dissolved in 0.1% aq. formic acid with 10-20% CH3CN added as needed for solubility. Solutions were concentrated on a Buchi Rotovapor R-210 with Chemglass fritted adapters. Compounds purified by HPLC or from Stability Studies (below) were concentrated by lyophilization using a Labconco Freezone 2.5 Plus. Analytical HPLC for purity determination was performed on a Waters system (1525 Binary Pump and 2487 Dual l Absorbance Detector). Column: Synergi 4m Fusion-RP by Phenomenex, 150 x 4.6 mm. Isocratic elution performed with 0 or 5% CH3CN in 0.1% aq. formic acid at 0.5 mL/min or 1.0 mL/min flow rate. Compounds were dissolved in 0.1% aq. formic acid for HPLC analysis. Glassware was dried while under high vacuum using a heat gun by Varitemp, Master Appliance Corp. (Model: Vt-750C) to heat glassware to >150 C and then charged with Ar. High vacuum from Thomas Industries Inc. (Welch Model 1400B-01, Serial EE121257). 1H and 13CNMR were recorded on a Bruker instrument operating at 300 and 75 MHz, respectively. NMR spectra were obtained as CDCl3, CD3OD, and (CD3)2SO solutions (reported in ppm), using residual solvent peaks in the 1H and 13C NMR spectra (CDCl3: 7.27, 77.23 ppm; CD3OD: 3.31, 49.15 ppm; and (CD3)2SO: 2.50, 39.51 ppm) as the reference standard, respectively. All J values are given in units of Hz. Reagents were purchased from commercial suppliers such as Sigma-Aldrich, Acros Organics, Fisher Scientific, Astatech, Wilmad LabGlass, and Pharmco-Aaper which were used without further purification. Anhydrous MeOH, DME, DMF and THF were purchased from EMD Chemicals (Drisolv line). Optical rotations were measured on a Rudolph Research Autopol III polarimeter (using sodium D line, 589 nm) and [alpha]D given in units of (degrees-mL)/(dm-g), and concentration (c) is reported in units of g/100 mL. HRMS analysis (TOF ES+) was performed on a Micromass Q-Tof Ultimamass spectrometer. pKa values were determined at Analiza using a pKa PRO Analyzer (AATI, Ames, IA), as follows: An electrophoretic separation was performed in parallel across 24 different pH values. The compounds were detected by UV at 214 nm. The average pH spacing between buffer points was 0.4 pH units (pH range of 1.7-11.2). Four consecutive capillary electrophoresis runs were performed for each compound. Norfloxacin was used as a standard. The total number of pKa values was predicted by relating mobility andcompound molecular weight using pKa Estimator software (AATI, Ames, IA). Melting points were obtained using a Mel-Temp apparatus and are uncorrected. Sonication was performed using a VWR Aquasonic Model 75T. Amino acid controls L-leucine, L-phenylalanine, L-arginine, L-tyrosine, L-tryptophan, L-glycine, L-isoleucine, and L-methionine were purchased from commercial suppliers and converted to their HCl salts (by suspension in dioxane and addition of 1.05 equiv. of 4N HCl in dioxane, followed by concentration in vacuo) prior to testing in cell assays. Gly-HA 12h was purchased from Alfa Chemistry. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping