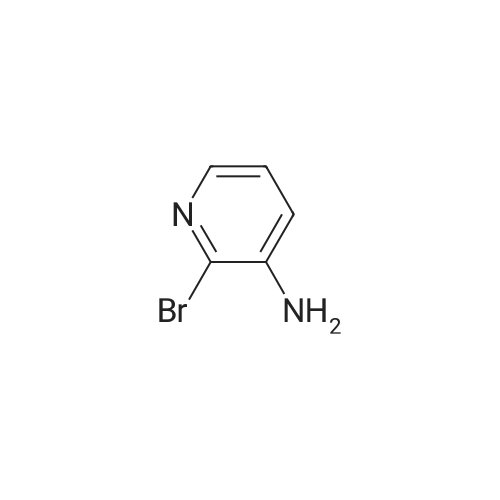
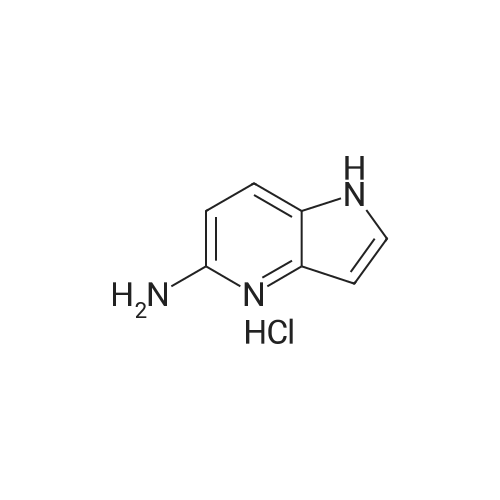
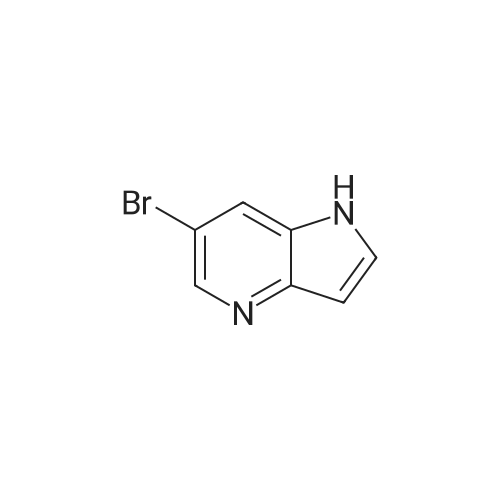
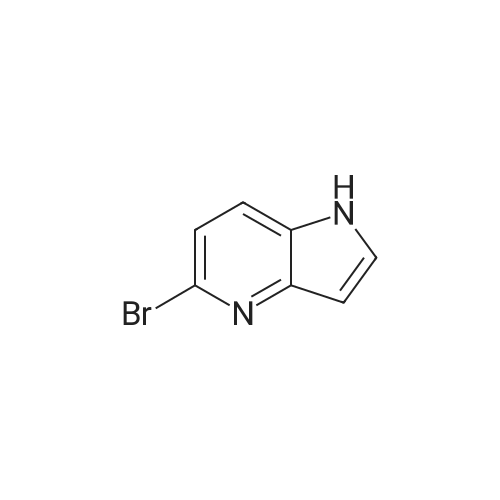
| 44% |
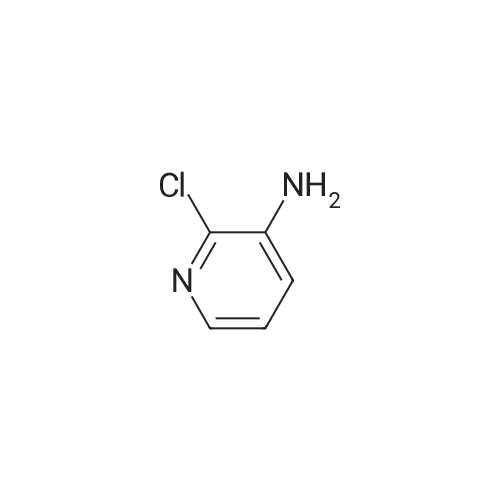
With potassium hydroxide; In dimethyl sulfoxide; at 20℃; for 12h; |
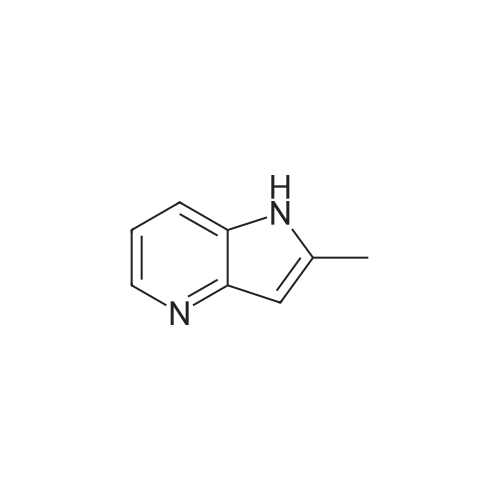
Example 2 (see general route 2, procedure E, F, C and D)2-(l-(4-chlorobenzyl)-2-methyl-lH-pyrrolo[3,2-b]pyridin-3-yl)-N-(2-methoxypyridin- 4-yl)-2-oxoacetamide (3) [00252] To a solution of 2-methyl-lH-pyrrolo[3,2-b]pyridine (74.4 mg, 0.563 mmol) and 4-chlorobenzyl chloride (0.0780 mL, 0.619 mmol) in DMSO (8 mL) at room temperature was added powdered potassium hydroxide (69.5 mg, 1.24 mmol). The reaction was stirred at room temperature for 12 hours, after which LCMS analysis indicated that the reaction was complete. The reaction mixture was diluted in water and extracted with dichloromethane (3 x 50 mL), dried (sodium sulfate), filtered and concentrated to a clear residue. Purification was achieved by silica gel chromatography using 30 to 90% ethyl acetate in hexanes over 80 minutes affording l-(4-chlorobenzyl)-2 -methyl- lH-pyrrolo[3,2-b]pyridine (64.2 mg, 0.250 mmol, 44% yield) as an off-white solid. NMR (400 MHz, CDC13) delta (ppm): 8.41 (dd, 1H), 7.41 (d, 1H), 7.24 (d, 2H), 7.01 (dd, 1H), 6.86 (d, 2H), 6.54 (s, 1H), 5.27 (s, 2H), 2.41 (s, 3H).[00253] To a solution of l-(4-chlorobenzyl)-2-methyl-lH-pyrrolo[3,2-b]pyridine (341 mg, 1.33 mmol) in dichloromethane (25 mL) was added aluminum trichloride (886 mg, 6.65 mmol). The mixture was stirred at room temperature for 20 minutes, after which ethyl oxalyl chloride (0.744 mL, 6.65 mmol) was added. The reaction mixture was stirred at room temperature for an additional three hours, after which it was poured over ice and extracted with ethyl acetate (3 x 50 mL), dried (sodium sulfate) filtered and concentrated to afford 2-(l- (4-chlorobenzyl)-2-methyl-lH-pyrrolo[3,2-b]pyridin-3-yl)-2-oxoacetic acid (76.5 mg, 0.233 mmol, 18% yield) an off-white solild. This material was used without any purification in the next step. The intended product of this reaction, ethyl 2-(l-(4-chlorobenzyl)-2-methyl-lH- pyrrolo[3,2-b]pyridin-3-yl)-2-oxoacetate, was observed in trace amounts and was not isolated from the reaction mixture. NMR (400 MHz, CDC13) delta (ppm): 8.44 (dd, 1H), 7.41 (dd, 1H), 7.20 (d, 2H), 7.04 (dd, 1H), 6.84 (d, 2H), 6.54 (s, 1H), 5.27 (s, 2H), 2.77 (s, 3H)[Carboxylic acid proton not detected in 1H NMR]. LCMS: 2.18 min, [ES]" found 327.10.[00254] To a solution of 2-(l-(4-chlorobenzyl)-2-methyl-lH-pyrrolo[3,2-b]pyridin-3- yl)-2-oxoacetic acid (76.5 mg, 0.218 mmol) in acetonitrile (7 mL) was added triethylamine (0.304 mL, 2.18 mmol), 2-methoxypyridin-4-amine (29.8 mg, 0.240 mmol), followed by a 50% ethyl acetate solution of T3P (972 mg, 1.53 mmol). The reaction was heated to 60 C for 2 hours, after which additional triethylamine (0.304 mL, 2.18 mmol) and T3P solution (972 mg, 1.53 mmol) were added. The reaction mixture was stirred at 60 C for 12 hours, after which it was diluted in water, extracted with ethyl acetate (3 x 50 mL), dried (sodium sulfate), filtered and concentrated to a residue. Purification was achieved by silica gel chromatography (Luknova 40g, 20 mL/min) using 10 to 60% ethyl acetate in hexanes over 60 minutes. 2-(l-(4-chlorobenzyl)-2-methyl-lH-pyrrolo[3,2-b]pyridin-3-yl)-N-(2- methoxypyridin-4-yl)-2-oxoacetamide (31 mg, 0.071 mmol, 33% yield) was isolated as a light-tan solid. NMR (400 MHz, CDC13) delta (ppm): 12.5 (br. s, IH), 8.55 (dd, IH), 8.08 (d, IH), 7.55 (dd, IH), 7.23-7.29 (m, 4H), 7.18 (dd, IH), 6.87 (d, 2H), 5.34 (s, 2H), 3.90 (s, 3H), 2.70 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping