Alternatived Products of [ 7305-71-7 ]
Product Details of [ 7305-71-7 ]
| CAS No. : | 7305-71-7 |
MDL No. : | MFCD00078317 |
| Formula : |
C4H6N2S
|
Boiling Point : |
- |
| Linear Structure Formula : | H2N(C3HNS)CH3 |
InChI Key : | GUABFMPMKJGSBQ-UHFFFAOYSA-N |
| M.W : |
114.17
|
Pubchem ID : | 351770 |
| Synonyms : |
|
Application In Synthesis of [ 7305-71-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 7305-71-7 ]
- 1
-
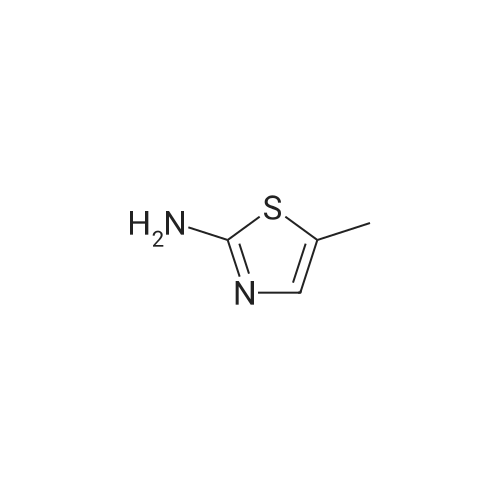 [ 7305-71-7 ]
[ 7305-71-7 ]

-
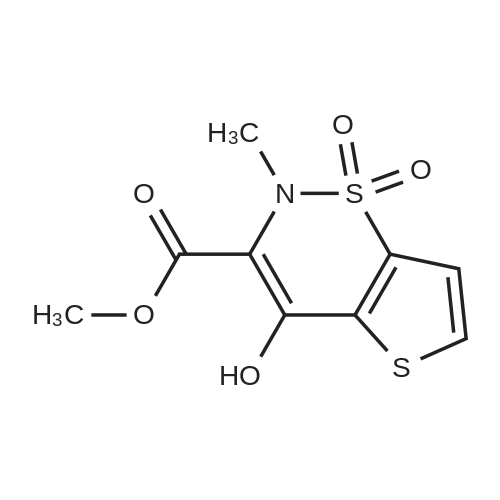 [ 59804-25-0 ]
[ 59804-25-0 ]

-
4-Hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1λ6-thieno[2,3-e][1,2]thiazine-3-carboxylic acid (5-methyl-thiazol-2-yl)-amide
[ No CAS ]
- 2
-
 [ 7305-71-7 ]
[ 7305-71-7 ]

-
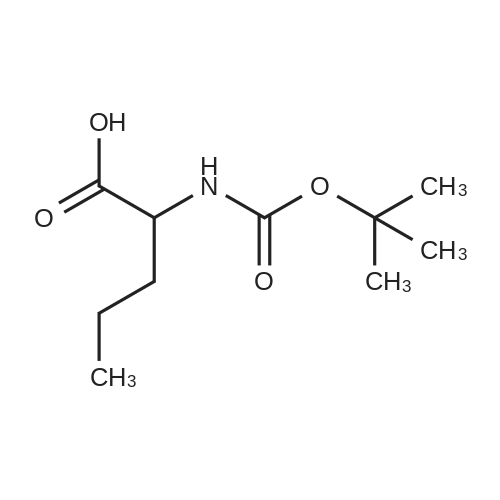 [ 521286-38-4 ]
[ 521286-38-4 ]

-
 [ 681143-01-1 ]
[ 681143-01-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; In dichloromethane; at 20℃; |
A mixture of 2-tert-butoxycarbonylamino-pentanoic acid (1.0 eq.), 2-amino-5-methyl thiazole (1.0 eq. ), HOBt (1.05 eq. ), EDC. HCI (1.2 eq. ) and a triethylamine (4 eq. ) in methylene chloride was stirred at room temperature overnight. The mixture was quenched with water and extracted with methylene chloride. The organic layer was washed with diluted HCI, separated, dried over sodium sulfate and filtered. The solvent was removed at reduced pressure to provide product. [M+1=314. 3, 1H] NMR (DMSO-d6) d 7.11 (s, 1H), 4.11 (m, 1H), 2.3 (s, 3H), 1.54 (m, 2H), 1.34 (t, 9H), 1.2-1. 4 (m, 2H), 0.83 (t, 3H) ppm. |
- 3
-
 [ 7305-71-7 ]
[ 7305-71-7 ]

-
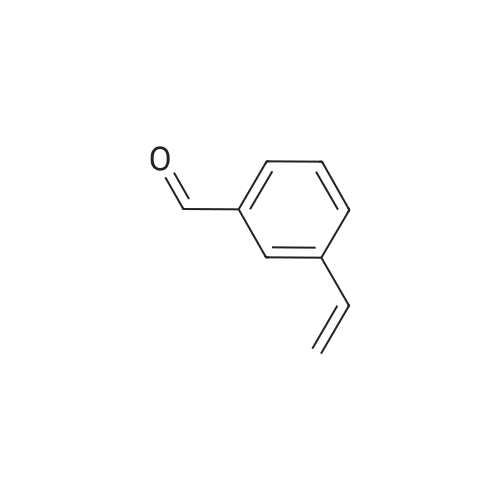 [ 19955-99-8 ]
[ 19955-99-8 ]

-
N-(3-vinylbenzylidene)-5-methyl-thiazol-2-amine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 72% |
With 2,6-di-t-butyl catechol; acetic acid; In ethanol; for 3h;Reflux; |
General procedure: A mixture of equal volumes of heterocyclic amine (0.02 mol), and vinylbenzaldehyde (1p and 1m) (0.02 mol) in the presence of some traces of 2,6-di-t-butyl catechol as the polymerization inhibitor, and 4-5 drops of glacial acetic acid used as reaction catalyst in 30 mL of absolute ethanol was refluxed for 3 h in water bath as shown in the Scheme 1. The resulting solution was concentrated in vacuum and cooled down in a freezer for 24 h. The precipitated product was filtered, washed with cold absolute ethanol and then dried. |
- 4
-
 [ 7305-71-7 ]
[ 7305-71-7 ]

-
 [ 117-21-5 ]
[ 117-21-5 ]

-
4-chloro-2-(5-methylthiazol-2-yl)isoindoline-1,3-dione
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 30% |
With acetic acid; at 110℃; for 4h; |
General procedure: The starting materials 1 and 2 were commercially available (Energy Chemical, Shanghai, China).Compound 2 (3.72 mmol) was added to a stirred solution of compound 1 (3.38 mmol) in glacial aceticacid (10 mL). The reaction mixture was then stirred at 110 C for 4 h. After completion of the reaction,the solvent was evaporated, and the residue was purified on a silica gel column chromatography andeluted with ethyl acetate/petroleum ether (bp 60-90 C) (1:3, v/v) to give compounds 3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










