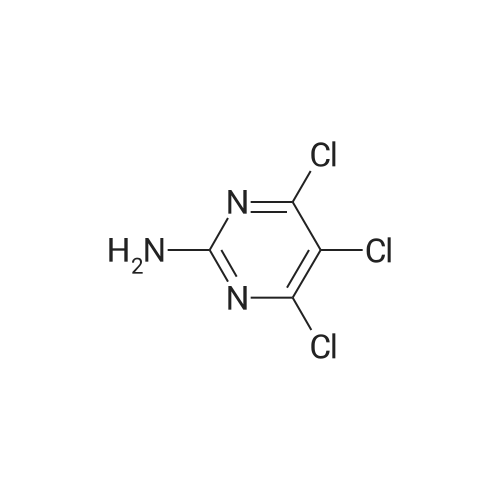
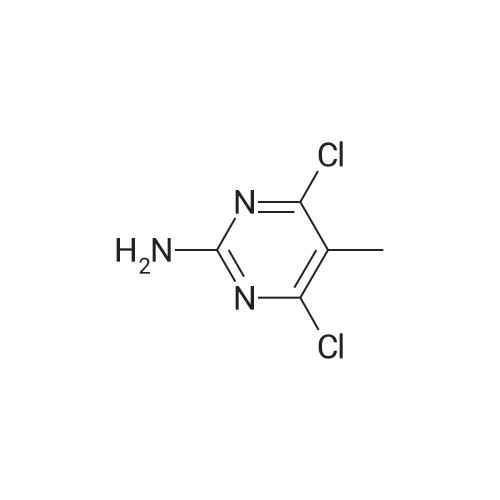
| 96% |
With hydrogenchloride; water In ethanol at 50℃; for 2 h; |
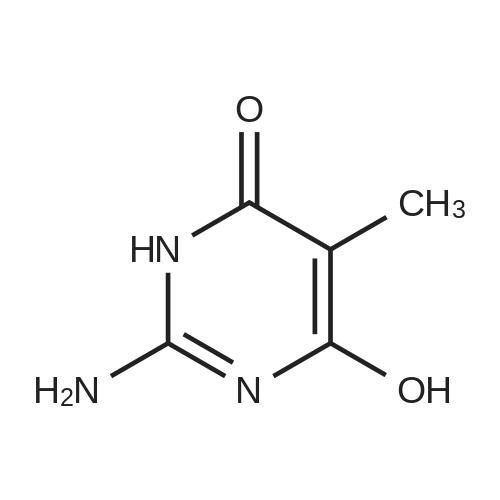
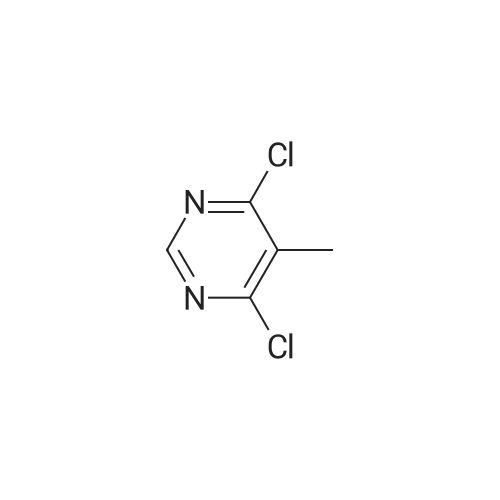
Substituted 4,6-dichloro-2-[(dimethylamino)methylene]amino}pyrimidines were subjected to deprotextion of the (dimethylamino)methylene protecting group from the amino group in position 2 of the pyrimidine ring. For this reaction, the method described in the literature [Nucleosides, Nucleotides Nucleic Acids 19(1 a2), 297-327, 2000] was used, innovatively modified in order to make it possible to merely filter the product from the reaction mixture. A flask was filled with 50 mmol of substituted 4,6-dichloro-2- [(dimethylamino)methylene] amino} -pyrimidine, 200 ml of 99percent ethanol and 20 ml of resultant HC1. The reaction mixture was subsequently warmed up to 50 °C for two hours, during which a crystalline product began to separate directly from the reaction mixture. After that, 300 ml of water were added and the reaction mixture was intensively stirred for 10 minutes while quantitatively yielding the desired product, which was subsequently sucked off and rinsed with 2x 50 ml of a water/ethanol mixture (1/1), lx 5percent NaHC0&3 aqueous solution and lx 50 ml of a water/ethanol mixture (1/1). The product was subsequently recrystallized in 99percent ethanol. After complete cooling to 0 °C, the separated white crystals were sucked off, rinsed with lx 50 ml of a water/ethanol mixture (1/1) and dried in a vacuum drier.2-amino-4,6-dichloro-5-methylpyrimidine (25)Yield: 8.53 g (96 percent of the theor. yield); m.p. 189-190 °C. NMR (DMSO-6): 7.26 bs, 2H, (NH2); 2.17 s, 3H, (Η-1'). 13C NMR (DMSO-6): 161.01 (C-4 and 6); 160.78 (C-2); 1 13.60 (C-5); 14.93 (C-Γ). For C5H5C12N3: calculated: 33.73 percent C, 2.83 percent H, 39.83 percent CI, 23.60 percent N; found: 33.53 percent C, 2.78 percent H, 40.02 percent CI, 23.42 percent N. MS (EI), m/z (percent): 177 and 179 [M+] (100). MS (ESI+), m/z (percent): 178 and 180 [M+H+] (100). |
| 1.26 g |
With hydrogenchloride In ethanol; water at 50℃; for 2 h; |
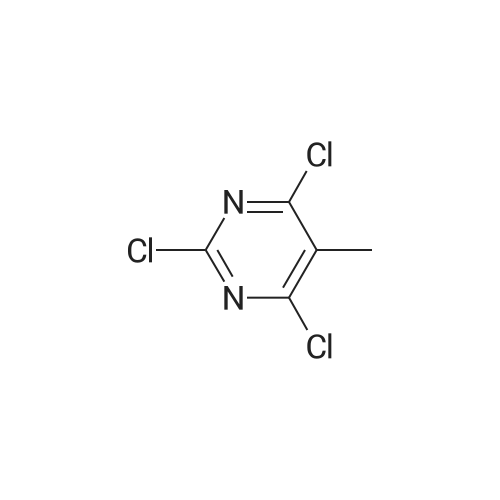
General procedure: Prior to the reaction, the starting 5-substituted 2-amino-4,6-dihydroxypyrimidine A2–A11 was dried in a vacuum drier at 80 °C and under 0.1 mbar for 1 day, because crystalline water increases the amount of the Vilsmeier–Haack–Arnold reagent required for full conversion. Subsequently, 5-substituted 2-amino-4,6-dihydroxypyrimidine (10 mmol) was suspended under inert atmosphere in a 2 M solution of the Vilsmeier–Haack–Arnold reagent (80 mmol, 40 mL) in chloroform. The reaction mixture was subsequently heated at reflux for 4 h, during which the starting material was completely dissolved. The reaction mixture was cooled to the room temperature, poured onto ice and rapidly neutralized with a saturated aqueous NaHCO3 solution. The obtained mixture was quickly transferred into a separatory funnel and immediately extracted with chloroform(3 x 20 mL). The organic fractions were combined together,dried over MgSO4, filtered through a 0.5 cm layer of silica gel and concentrated down on a rotary evaporator. This crude residue of 5-substituted 4,6-dichloro-2-[(dimethylamino)methylene]amino}pyrimidine was dissolved in the mixture of 99 percent ethanol (20 mL) and 37 percentaqueous HCl (2 mL). The reaction mixture was heated at 50 °C for 2 h, during which a crystalline product began to precipitate directly from the reaction mixture. After that, water (30 mL) was added and the reaction mixture was intensively stirred for 10 min. The precipitated product was filtered off and washed with a water/ethanol mixture (1/1,2 x 10 mL), 5 percent aqueous solution of NaHCO3 (10 mL) and a water/ethanol mixture (1/1, 10 mL). The product was subsequently recrystallized from aqueous ethanol, filtered off, washed with a water/ethanol mixture (1/1, 10 mL) and dried under high vacuum. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping