|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 0 - 20℃; for 1.5h; |
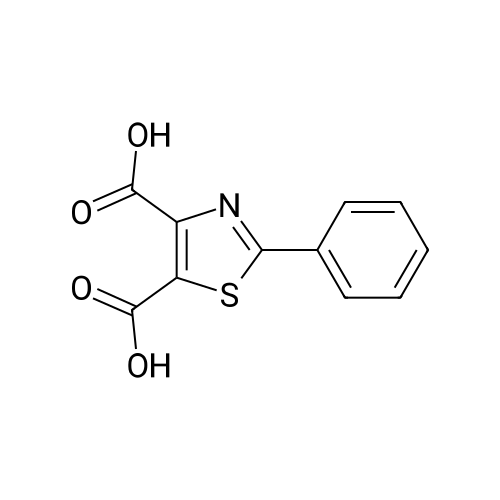
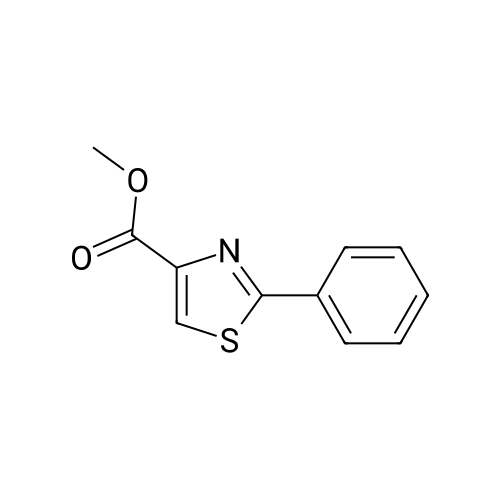
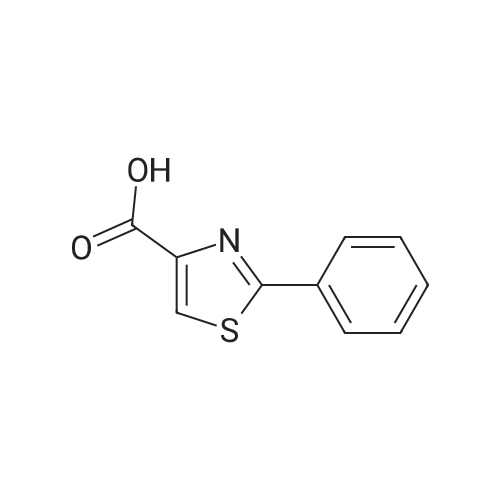
(Table 8) To a solution of 250 mg of <strong>[7113-10-2]2-phenylthiazole-4-carboxylic acid</strong> in 15 ml of dichloromethane was added a catalytic amount of N,N-dimethylformaide, and then 175 mul of oxalyl chloride was dropped thereinto with ice cooling.. The mixture was stirred for one hour with ice cooling and for 30 minutes at room temperature, and the solvent was evaporated therefrom.. The residue was dissolved in 10 ml of acetonitrile, and the solution was dropped, with ice cooling, into a solution of 250 mg of methyl (Z)-(4,4-difluoro-2,3,4,5-tetrahydro-1H-1-benzoazepin-5-ylidene)acetate and 170 mul of triethylamine in 15 ml of acetonitrile.. The reaction solution was stirred for two hours at room temperature and for three hours at 50 C., and 100 ml of a saturated aqueous solution of sodium bicarbonate was added.. This was extracted with ethyl acetate, followed by washing with a saturated aqueous solution of NaCl. After drying over magnesium sulfate, the solvent was evaporated therefrom.. The oily residue (500 mg) was dissolved in 10 ml of methanol, 3 ml of a 1N aqueous solution of sodium hydroxide was added, and the mixture was stirred at room temperature for 18 hours.. After subjecting the reaction solution to evaporation, water was added to the residue, and the mixture was washed with ethyl acetate.. The aqueous solution was acidified with 10 ml of 1N hydrochloric acid, extracted with ethyl acetate and washed with a saturated aqueous solution of NaCl. After drying over magnesium sulfate, the solvent was evaporated therefrom.. The residue (400 mg) was dissolved in 10 ml of tetrahydrofuran, then 210 mg of 1-hydroxybenzotriazole, 280 mg of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide monohydrochloride and 210 mul of 4-(2-aminoethyl)morpholine were added thereto, and the mixture was stirred at room temperature for 18 hours.. To the reaction solution was added 50 ml of a saturated aqueous solution of sodium bicarbonate, and the mixture was extracted with ethyl acetate.. The organic layer was washed with a saturated aqueous solution of NaCl and dried over magnesium sulfate, and the solvent was evaporated therefrom.. The residue was purified by silica gel column chromatography (eluding with ethyl acetate-methanol) and crystallized from ethanol to give 90 mg of (Z)-[4,4-difluoro-1-(2-phenylthiazole-4-carbonyl]-2,3,4,5-tetrahydro-1H-1-benzoazepin-5-ylidene]-N-(2-morpholinoethyl)acetamide as a colorless powder. |
|
With thionyl chloride; In benzene; at 20℃; for 1h;Heating / reflux; |
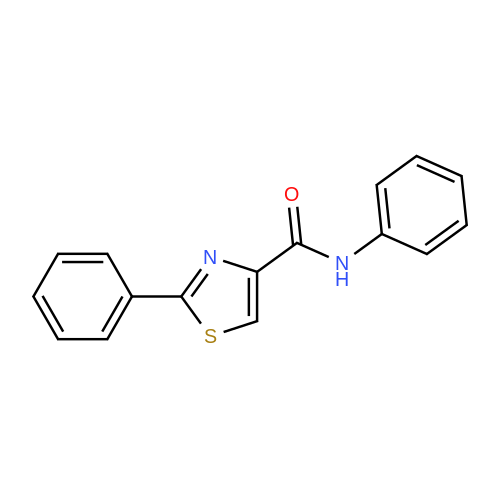
To <strong>[7113-10-2]2-phenylthiazole-4-carboxylic acid</strong> (70 mg) in 5 ML benzene was added thionyl chloride(0.075 ML) at room temperature.. The mixture was heated under reflux for an hour The mixture was cooled and evaporated under reduced pressure.. To the mixture added was dichloromethane (10 ml) followed by 3-(imidazol-1-yl)aniline (54 mg) and triethylamine (0.1 ml).. The mixture was stirred at room temperature for an hour.. The mixture was washed with a saturated aqueous sodium bicarbonate solution, dried with sodium sulfate and evaporated.. The residue was recrystallized from diisopropyl ether/ethyl acetate to give N-(3-(imidazol-1-yl)phenyl)-2-phenylthiazole-4-carboxamide. mp: 131-134 C. IR (nujol, nu): 1665 cm-1 NMR (DMSO-d6, delta): 7.14 (1H, s), 7.42 (1H, d, J=9 Hz), 7.45-7.60 (4H, m), 7.72 (1H, s), 7.94 (1H, d, J=8 Hz), 8.10-8.25 (4H, m), 8.54 (1H, s), 10.41 (1H,s) Mass m/z: 347 (M+1). |
|
With oxalyl dichloride;N,N-dimethyl-formamide; In dichloromethane; at 0 - 20℃; |
Example 43 To a solution of 821 mg of <strong>[7113-10-2]2-phenyl-1,3-thiazole-4-carboxylic acid</strong> in 30 ml of methylenechloride were added dropwise 520 mul of oxalyl chloride and 15 mul of DMF at 0C, followed by stirring at room temperature for 3 hours. From this reaction liquid, 300 mul portion was collected, a solution of 8.3 mg of 2-(methylsulfonyl) aniline hydrochloride and 11 mul of triethylamine in 200 mul of methylenechloride was added thereto at room temperature, followed by stirring overnight. To the reaction liquid were added 100 mg of PS-Isocyanate (Argonaut Technologies, Inc.), 75 mg of PS-Trisamine (Argonaut Technologies, Inc.), and 1 ml of DMF, followed by stirring at room temperature overnight, and the insoluble materials were filtered. The filtrate was concentrated under reduced pressure and the obtained residue was purified by preparative high performance liquid chromatography (methanol-aqueous 0.1% formic acid solution) to prepare 8.4 mg of N-[2-(methylsulfonyl)phenyl]-2-phenyl-1,3-thiazole-4-carboxamide. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping