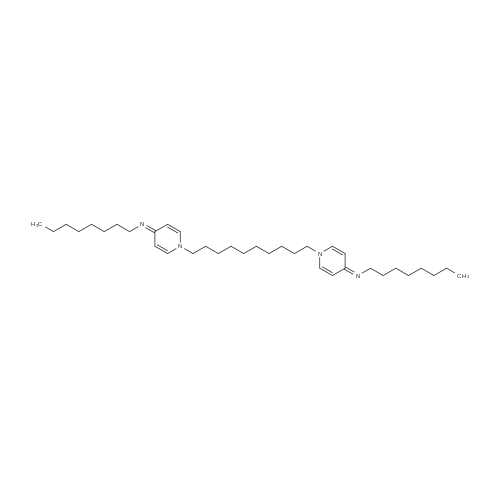
Electrostatically hindered diffusion for predictable release of encapsulated cationic antimicrobials
Viktor Eriksson
;
Erik Nygren
;
Romain Bordes
, et al.
RSC Pharm.,2024,1,47-56.
DOI:
10.1039/d3pm00025g
More
Abstract: A common challenge in infection control is uncontrolled and unpredictable rapid release of antimicrobials – with ramifications on antimicrobial resistance (AMR) development and pollution – that makes it difficult to determine appropriate dosage levels and treatment times. An important class of antimicrobials is surface-active cationic substances, whose charge can be exploited for manipulating both their encapsulation and controlled release. As a proof of concept, the cationic antimicrobial octenidine dihydrochloride (OCT) was encapsulated in a microcapsule matrix of poly(D,L-lactide-co-glycolide) (PLGA) bearing anionic carboxylate end groups. The strong PLGA–OCT interaction was verified by infrared spectroscopy and by comparing the release of OCT to its uptake into empty microcapsules. By expanding a Fickian diffusion model, the binding event was estimated to result in a 10-fold reduction in effective diffusivity resulting in a sustained release maintained for several months. Using this model, the impacts of temperature and release medium solubilizers were globally examined to improve predictability. By exceeding the glass transition temperature of hydrated PLGA, the diffusional release was significantly faster at 37 °C with a diffusivity 200 times that at room temperature. The addition of solubilizers increased the OCT partitioning towards the aqueous phase without affecting its diffusivity.
Purchased from AmBeed:
70775-75-6

Contribution of RND-Type Efflux Pumps in Reduced Susceptibility to Biocides in Acinetobacter baumannii
Christina Meyer
;
Kai Lucaβen
;
Stefanie Gerson
, et al.
Antibiotics,2022,11(11):1635.
DOI:
10.3390/antibiotics11111635
PubMed ID:
36421279
More
Abstract: Bacterial efflux pumps are among the key mechanisms of resistance against antibiotics and biocides. We investigated whether differential expression levels of the RND-type efflux pumps AdeABC and AdeIJK impacted the susceptibility to commonly used biocides in multidrug-resistant Acinetobacter baumannii. Susceptibility testing and time–kill assays of defined laboratory and clinical A. baumannii strains with different levels of efflux pump expression were performed after exposure to the biocides benzalkonium chloride, chlorhexidine digluconate, ethanol, glucoprotamin, octenidine dihydrochloride, and triclosan. While the impact of efflux pump expression on susceptibility to the biocides was limited, noticeable differences were found in kill curves, where AdeABC expression correlated with greater survival after exposure to benzalkonium chloride, chlorhexidine digluconate, glucoprotamin, and octenidine dihydrochloride. AdeABC expression levels did not impact kill kinetics with ethanol nor triclosan. In conclusion, these data indicate that the overexpression of the RND-type efflux pumps AdeABC and AdeIJK contributes to the survival of A. baumannii when exposed to residual concentrations of biocides.
Keywords:
efflux ;
biocide ;
disinfectant ;
RND-type efflux pump ;
Acinetobacter baumannii
Purchased from AmBeed:
70775-75-6

Antiseptic microspheres embedded in nonwoven ?ber materials
Jakobsen, Petrus
;
Chalmers University of Technology,2019.
More
Abstract: A challenge facing the medical sector is the treatment of non-healing chronic wounds and the threat of multidrug resistant bacteria strains. A potential solution to these challenges would be to create wound care bandages with better durability and reduced risk of cultivating multidrug resistant bacteria strains. In this thesis, a solution is proposed by incorporatin biocompatible polymer microspheres containing the antiseptic agents octenidine dihydrochloride (OCT) and benzalkonium chloride (BAC) in a nonwoven cellulose material. The spheres were formulated using the solvent evaporation method, where the influence of emulsifier and homogenization on the sphere stability and size were investigated. It was found that a higher concentration of emulsifier counteracted aggregation of the spheres and that stirring for a short time gave the largest spheres. The mainly used polymers were poly(lacticco-glycolic acid) (PLGA), poly(L-lactic acid) (PLLA) and bis–(2–carboxyphenyl) adipate polyanhydride (SASanhydride). The influence of the active substance on the interfacial tension was investigated with optical tensiometry by measuring the interfacial tension of dichloromethane(DCM) droplets with different concentrations of active substance to a water phase. Both OCT and BAC are surface active agents and had a considerable effect on the interfacial tension between the DCM droplets and the surrounding aqueous medium. The reduced interfacial tension due to the surface activity of BAC and in particular OCT resulted in smaller spheres. The formulated microspheres with OCT were subsequently incorporated into a nonwoven fiber material using solution-blow spinning. In this process, a solution was created containing an ionic liquid and microcrystalline cellulose where the spheres containing the active substance were dispersed. The ionic liquid, in this case, 1- ethyl-3-methylimidazolium acetate(EmimAc) was used to dissolve the cellulose. To determine the amount of active substance required in the nonwoven fiber to achieve an antimicrobial effect, the minimal inhibitory concentration (MIC) was used. At MIC the bacteria population in the wound is contained. With the proposed fabrication process, the incorporated amount of BAC in the fiber is assumed to lead to a concentration below the MIC while the concentration may be high enough for fibers with OCT containing spheres. To increase the BAC content in the fiber, a possible solution could be impregnation with BAC combined with microspheres containing BAC. A distribution coefficient, KOCT = 6.23 and KBAC = 2.86 for the active substances was determined with respect to the distribution between the polymer and water phase. The result indicated that there was a risk of leakage of active substance from the polymer spheres to the water phases in the emulsion and particularly in the coagulation and wash bath used in the solution-blow spinning. Solubility tests for OCT were done where it was found to have a higher solubility then values found in the literature.
Keywords:
PLGA ;
PLLA ;
SASanhydride ;
microspheres ;
solution-blow spinning ;
solvent evaporation ;
emulsion ;
interfacial tension ;
distribution coefficient ;
octenidine dihydrochloride ;
benzalkonium chloride
...More
Purchased from AmBeed:
70775-75-6


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping