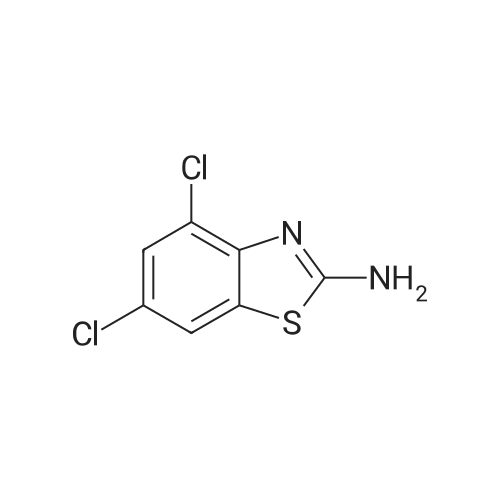
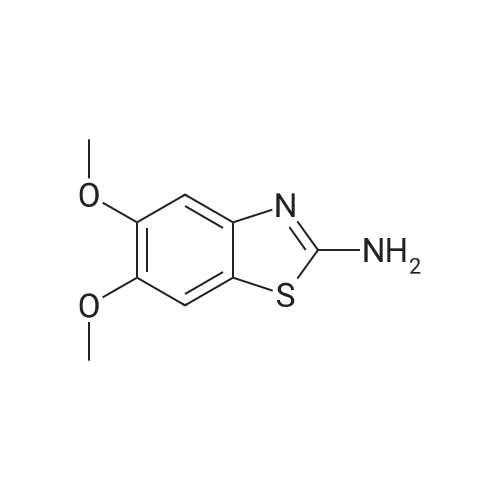
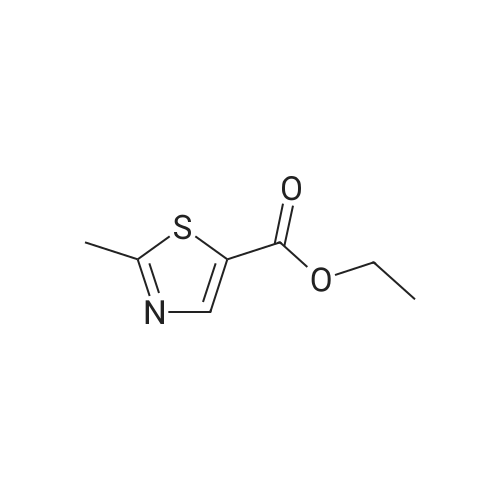
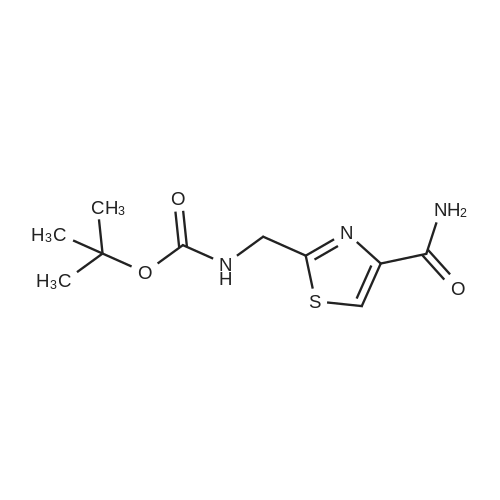
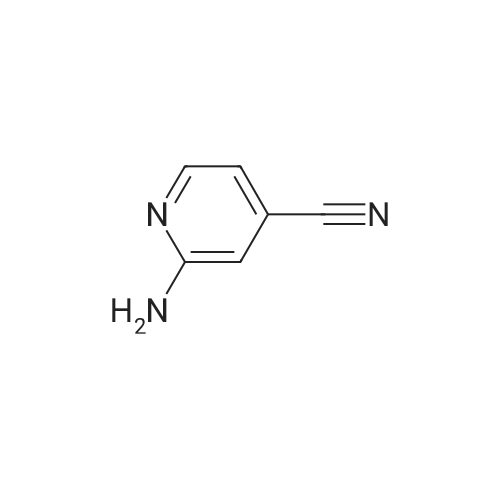
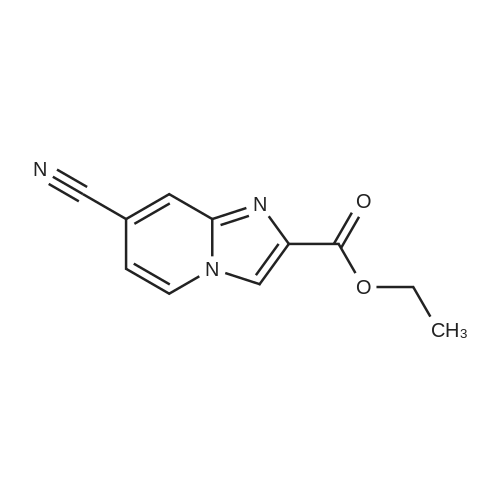
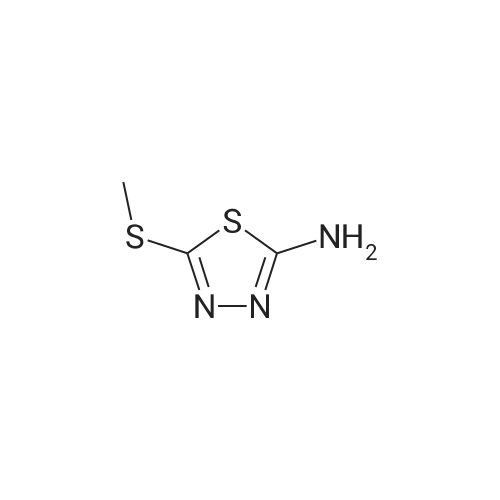
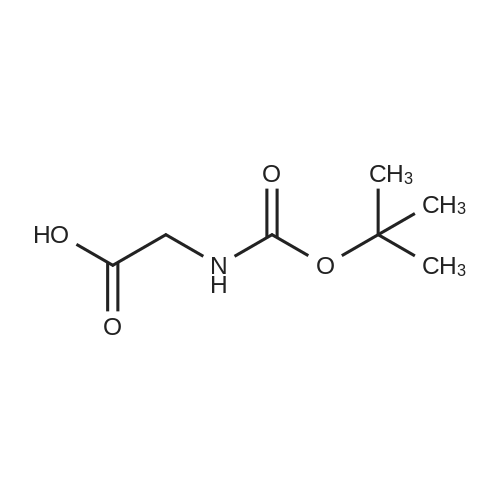
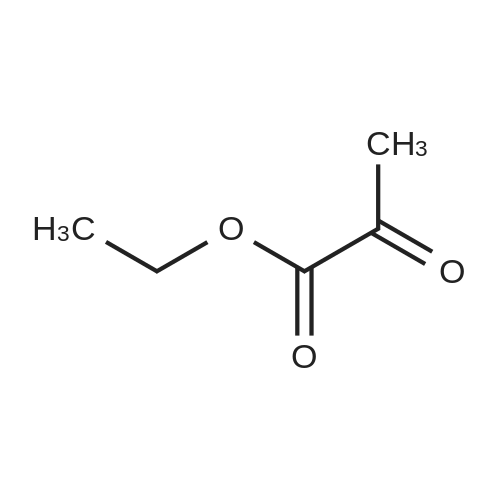
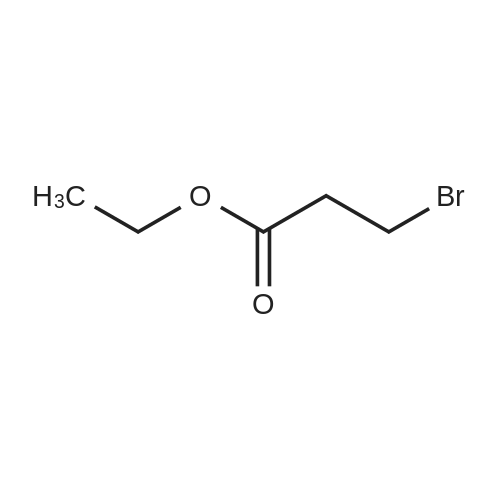
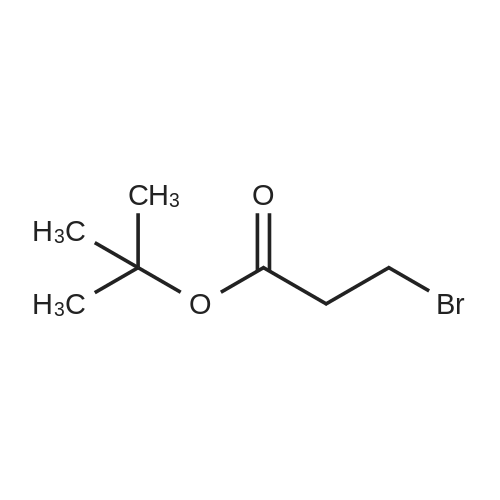
| 67% |
|
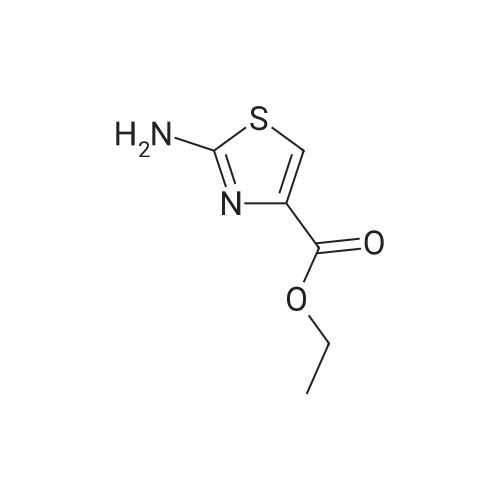
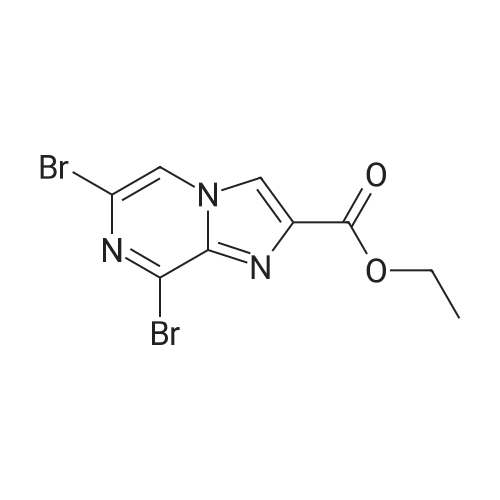
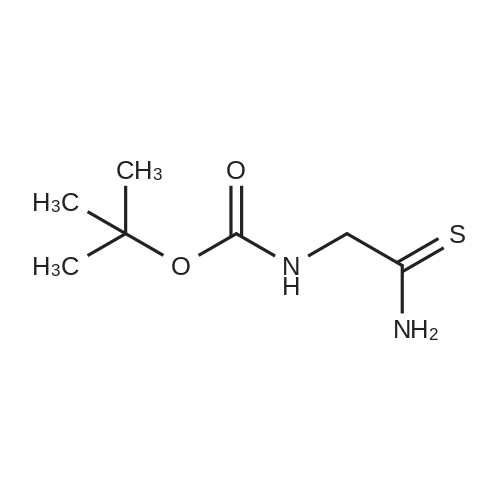
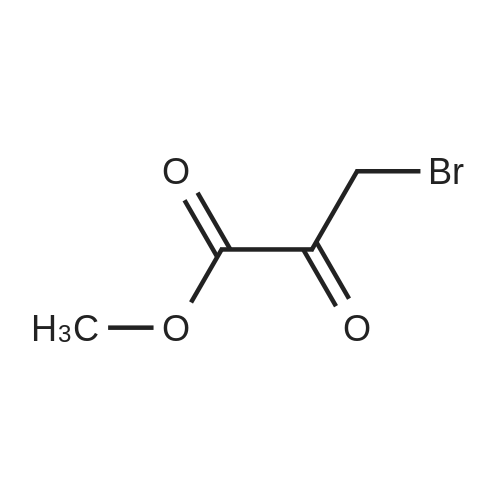
To compound 387 (6.00 g, 31.5 mmol) dissolved in CH2CI2 (150 mL) was added ethyl bromopyruvate (6.76 g, 4.4 mL, 34.7 mmol). Stirred at room temperature for 5 h then concentrated. Added 3 A sieves (6 g) and EtOH (150 mL) and refluxed for 16 h. Filtered and concentrated to give a dark foam. Dissolved foam in 1:1 CH2CI2:EtOH (100 mL) and added triethylamine (6.40 g, 8.8 mL, 63.1 mmol) and tBOC anhydride (7.60 g, 34.7 mmol). Stirred at room temperature for 5 h then concentrated. Added 0.25 N NaOH (100 mL), extracted with CH2CI2, dried combined organic extracts (MgS04), filtered, and concentrated. Purified by silica gel chromatography (eluant: 10% EtOAc- CH2Cl2 to 30% EtOAc-CH2Cl2) to give 6.00 g (67%) of the product 388 as a brown oil. MS m/e: 287 (M+H). For n=2: MS m/e: 301 (M+H) |
| 65% |
With pyridine; In ethanol; for 24h;Inert atmosphere; Reflux; |
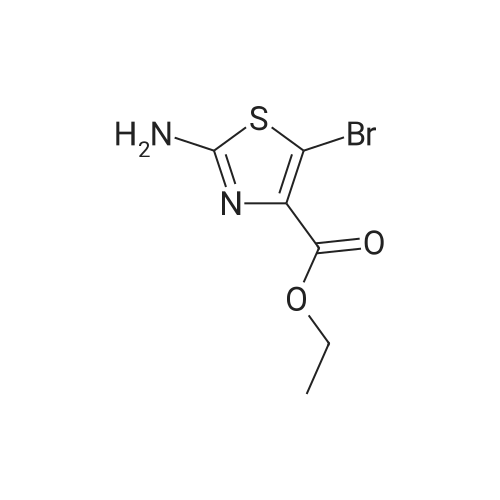
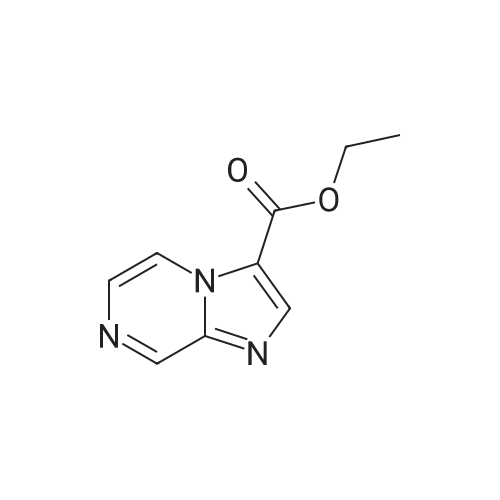
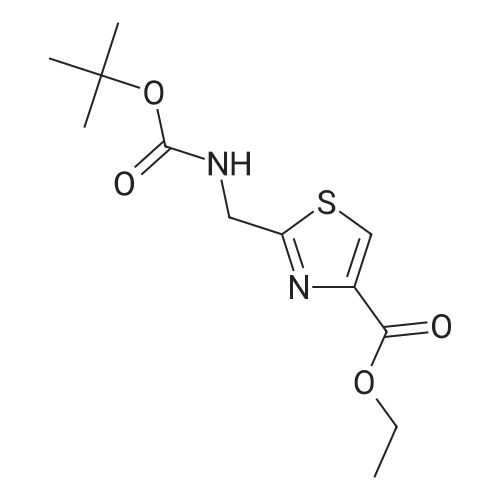
Ethyl bromopyruvate (1.75 mL, 14.0 mmol) and pyridine (1.69 mL, 21.0 mmol) were added to a solution of 8 (1.33 g, 7.0 mmol) in dry EtOH (60 mL) under N2 atmosphere. The reaction mixture was refluxed 24 hs. The volatile components were removed in vacuo. The resulting residue was dissolved in EtOAc (40 mL), washed with water (30 mL) and brine (30 mL). The organic layer was dried with MgSO4, filtered and concentrated in vacuo. Purification by column chromatography afforded 5 (1.31g, 65%) as a brown solid. Rf = 0.5 (hexane/ EtOAc, 1:1); 1H NMR (400MHz, CDCl3) delta 1.39 (t, 3H, J = 7,1 Hz), 1.46 (s, 9H), 4.14 (q, 2H, J = 7.1 Hz), 4.64 (d, 2H, J = 6.3 Hz), 5.31 (bs, 1H), 8.11 (s, 1H5). 13C NMR (100MHz, CDCl3) delta 14.3, 28.3 (3C), 42.4, 61.5, 80.5, 127.9, 146.9, 155.6, 161.3, 170.0. |
|
In ethanol; at 20 - 25℃; for 5h;Product distribution / selectivity; |
a) Boc-2-Aminomethylthiazole-4-carboxamide [00073] At 10 C., ethyl bromopyruvate (386 g, 1.98 mol) was added dropwise to a solution of Boc-glycinethioamide (370 g, 1.94 mol) in 3.9 liters of ethanol, and the mixture was then stirred at 20-25 C. for 5 h, after which 299 ml of a 25% strength aqueous ammonia solution were added. [00074] From 940 ml of this mixture (corresponds to 19.9% of the total volume), 380 ml of ethanol were distilled off, a further 908 ml of a 25% strength aqueous ammonia solution were added and the mixture was stirred at 20-25 C. for 110 h. The mixture was cooled to 0 C. and the solid was filtered off, washed twice with water and dried. This gave 60.1 g of the BOC-protected thiazole carboxamide of an HPLC purity of 97.9 area %, which corresponded to a yield over these two steps of 60.5%. [00075] 1H-NMR (DMSO-d6, in ppm): 8.16 (s, 1H, Ar-H), 7.86 (t, broad, 1H, NH), 7.71 and 7.59 (2×s, broad, 1H each, NH2), 4.42 (d, 2H, CH2), 1.41 (s, 9H, tert-butyl). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +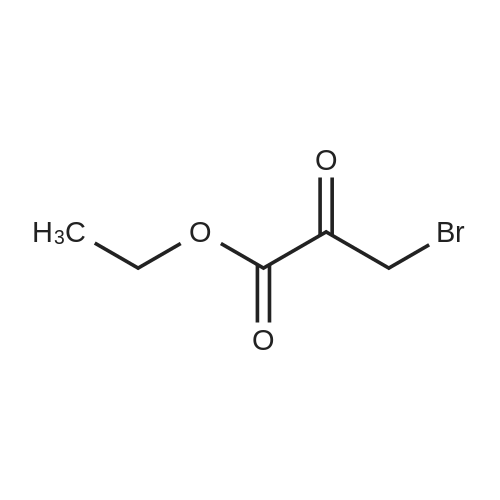

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping