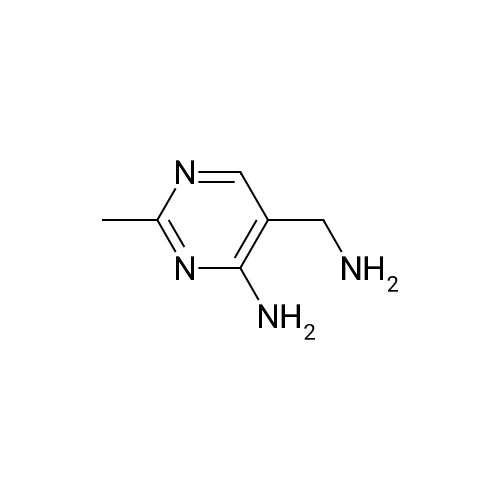
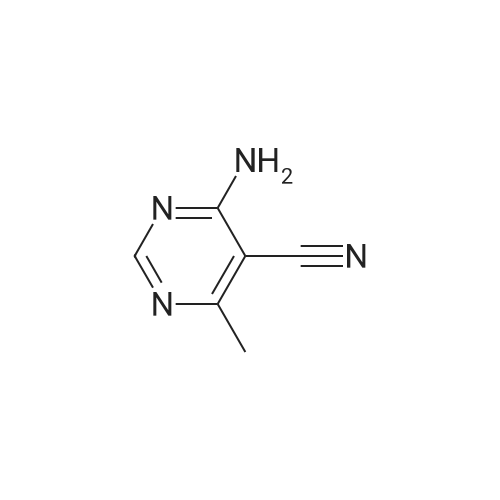
| 83% |
With formic acid; nickel; In water; for 0.25h;Reflux; |
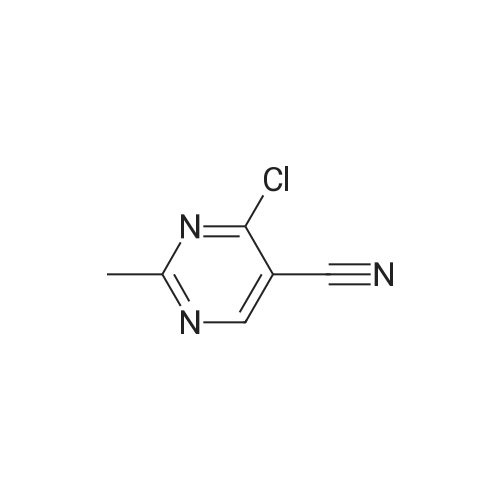
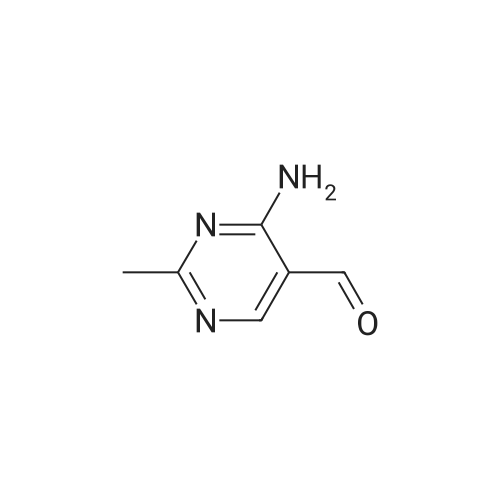
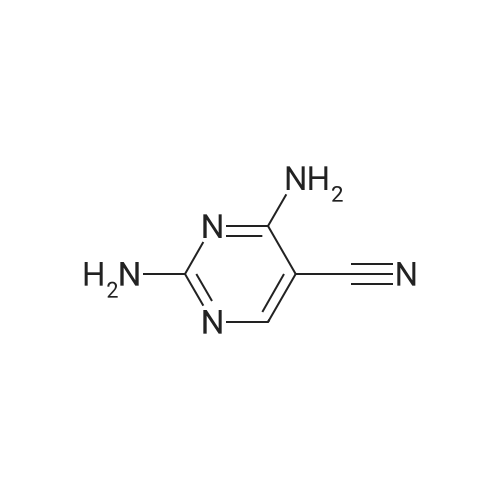
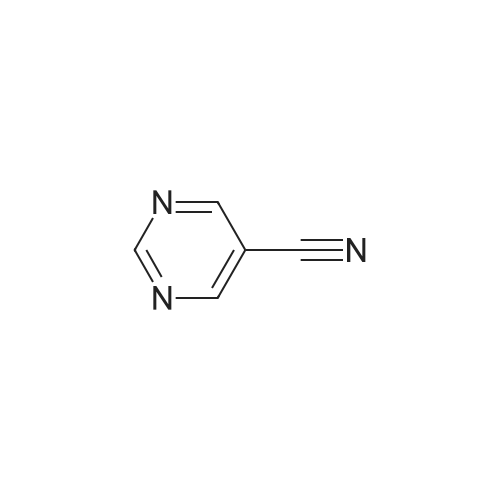
(0273) To a solution of the compound of Reference Example 22 (50.0 g, 373 mmol) in formic acid (150 mL) were added water (65 mL) and Raney nickel (50 g). The mixture was heated under reflux for 15 minutes, cooled to room temperature, and filtrated through Celite, and then 28% ammonia water (220 mL) was added thereto under ice cooling. The mixture was stirred under ice cooling for 1 hour, and the precipitate was collected by filtration. The filter cake was washed with water (30 mL) and chloroform (30 mL×2), and dried in vacuo. Furthermore, the filtrate was extracted with chloroform (200 mL) nine times, and the combined organic layer was concentrated. The resulting concentrated residue and the above-obtained filter cake were mixed, chloroform (70 mL) was added thereto, the mixture was stirred at room temperature for 30 minutes, hexane (210 mL) was added dropwise thereto over 10 minutes, and the mixture was stirred at room temperature for additional 1 hour. The precipitate was collected by filtration, washed with hexane/chloroform (3/1, 28 mL), and dried in vacuo to give the title compound (42.6 g, 83%). 1H-NMR (400 MHz, CDCl3) delta: 2.57 (3H, s), 5.98 (1H, brs), 8.15 (1H, brs), 8.57 (1H, s), 9.86 (1H, s). |
| 83% |
With formic acid; In water; for 0.25h;Reflux; |
Compound of Reference Example 22 (50.0g, 373mmol) was added to the formic acid solution (150mL) and water (65mL) Raney nickel (50g). After heating to reflux for 15 min, cooled to room temperature, filtered through celite, under ice-cooling 28% aqueous ammonia (220 mL) was added, after stirring for 1 hour under ice-cooling, the precipitate was collected by filtration. The filtrate quality goods and washed with water (30 mL) and chloroform (30 mL × 2 times) followed by drying under reduced pressure. Further, the filtrate was extracted 9 times with chloroform (200 mL), the organic layer was concentrated. The combined quality goods braze obtained this concentrated residue before, chloroform (70 mL) was added, after stirring for 30 minutes at room temperature, hexane (210 mL) was added dropwise over 10 minutes, further stirred at room temperature for 1 hour. Thereafter, the precipitate was collected by filtration, washed with hexane / chloroform (3 / 1,28mL), the title compound was dried under reduced pressure (42.6g, 83%) was obtained. |
| 70% |
With sulfuric acid; palladium 10% on activated carbon; hydrogen; In water; at 20℃; for 16h; |
To a solution of 51 (700 mg, 5.2 rnrnoi) in water and H2S04 is added Pd/C (10 wt. %, 543 mg). After stirring at room temperature under hydrogen for 16 hours, the mixture is filtered through a. pad of? Celite and washed with water. The filtrate is treated with ammonium hydroxide, and the solid is collected by filtration and dried to give 52 (500 mg, 70%). (MS: [M±HI 1381) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping