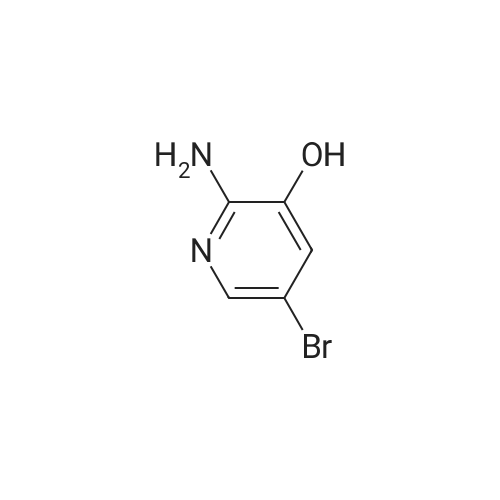
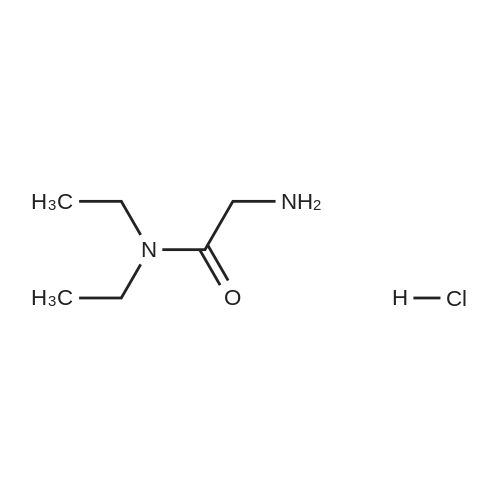
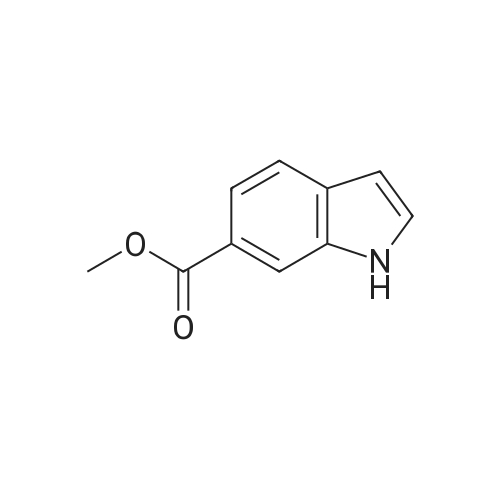
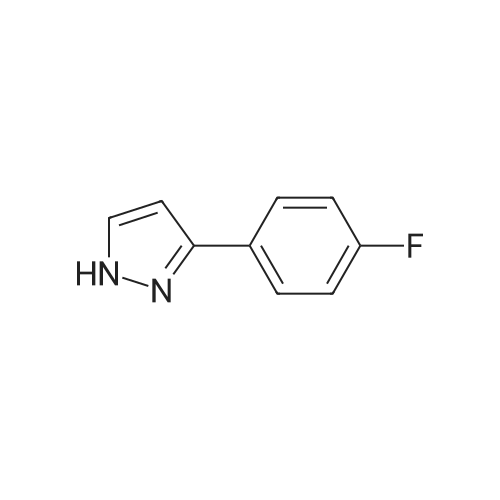
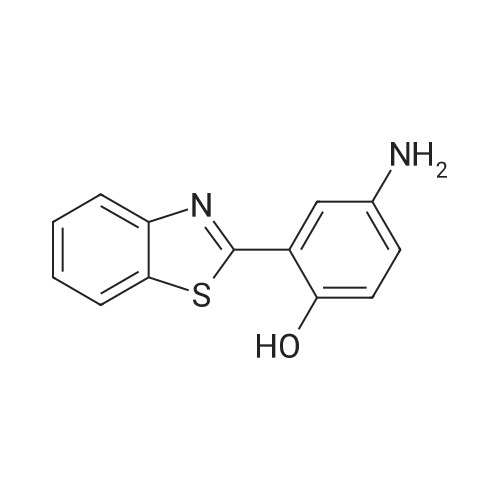
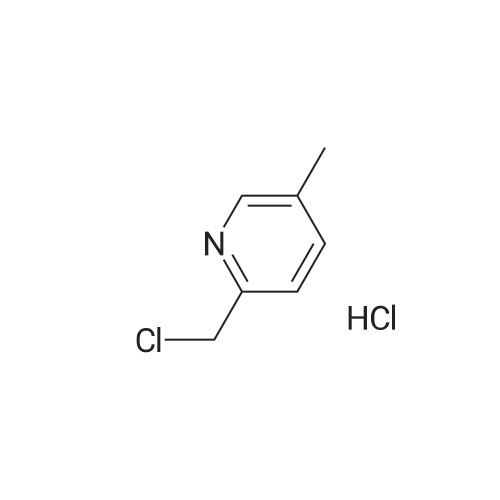
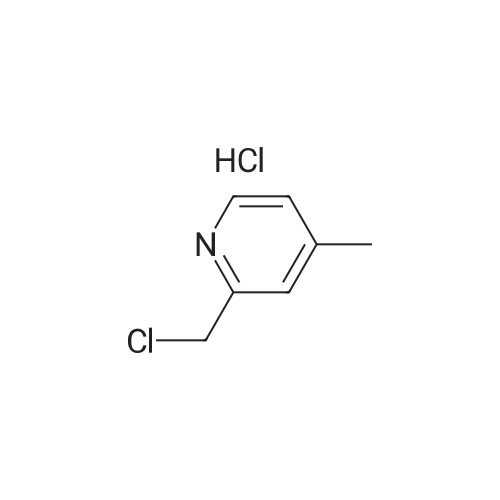
| 98% |
Stage #1: With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5 h;
Stage #2: With potassium iodide In N,N-dimethyl-formamide at 60℃; for 12 h; |
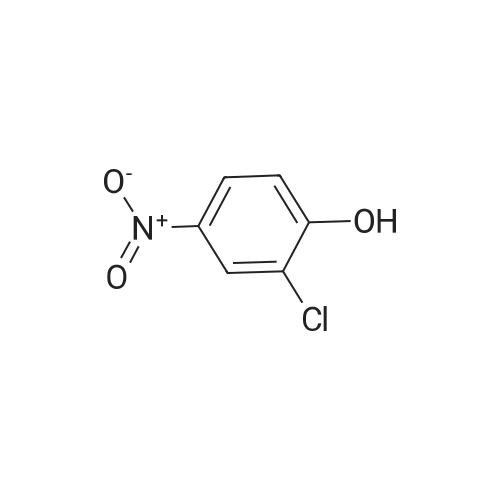
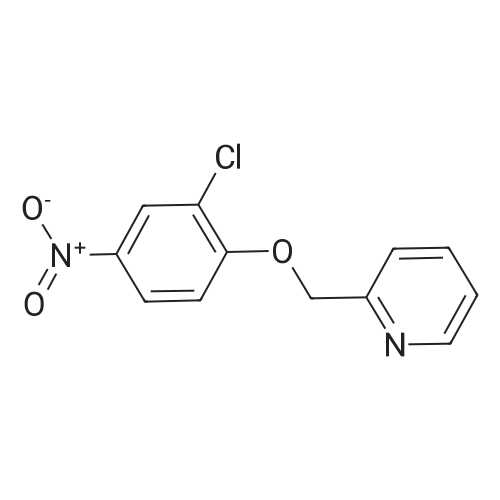
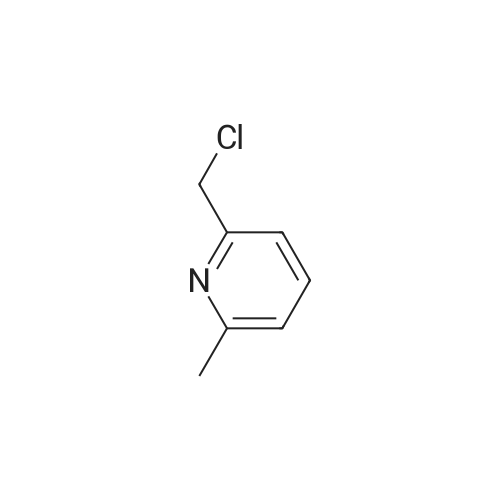
2-(Chloromethyl)pyridine hydrochloride (16.4 g, 0.1 mol) and K2CO3 (27.6 g, 0.2 mol) were suspended in DMF (100 mL) and stirred at room temperature for 30 min. 2-Chloro-4-nitrophenol (17.4 g, 0.1 mol) and KI (0.83 g, 5 mol percent) were added in and the reaction mixture was stirred at 60 °C for 12 h. The reaction suspension was diluted with water (400 mL) and the resulting solid was filtered, washed with water and dried to give 2-((2-chloro-4-nitrophenoxy)methyl)pyridine (26 g, 98percent) as a white solid. Mp 149.2–149.9 °C; MS-EI (m/z): 92, 229, 263(M+). |
| 74% |
With potassium carbonate; sodium iodide In acetonitrileReflux |
Step 1:
2-((2-chloro-4-nitrophenoxy)methyl)pyridine
2-chloro-4-nitrophenol (3.4 g, 20 mmol), 2-(chloromethyl)pyridine hydrochloride (3.4 g, 21 mmol), potassium carbonate (3.3 g, 24 mmol) and sodium iodide (3.0 g, 20 mmol) were refluxed in acetonitrile (30 mL) overnight.
The reaction mixture was poured into 100 mL of H2O, extracted with ethyl acetate.
The organic phase was washed with saturated brine, dried, evaporated with rotary evaporator, to obtain the crude product.
The crude product was washed with petroleum ether, filtered and dried, and the compound shown in the title (3.9 g, 74percent) was obtained.
1H NMR (CDCl3): δ 8.63 (1H, d, J=4.8 Hz), 8.34 (1H, d, J=2.8 Hz), 8.16-8.14 (1H, m), 7.79-7.76 (1H, m), 7.62-7.60 (1H, m), 7.31-7.27 (1H, m), 7.11 (1H, d, J=9.2 Hz), 5.49 (2H, s). |
| 52% |
With caesium carbonate; sodium iodide In acetonitrile at 60℃; for 5 h; |
2-CHLORO-4-NITRO PHENOL 10G (57.6 MMOL, 1EQ), 2-PYCOLYL CHLORIDE hydrogen chloride 9.45g (57.6 mmol, 1 eq) cesium carbonate 41.3 (126.8 mmol, 2.2 eq) and sodium iodide 8. 64G (57.6 mmol, 1 eq) were suspended in 200 mL acetonitrile. The reaction mixture was stirred at 60°C for 5h. The resulted suspension was filtered and washed with 400 mL water, YIELDING 2- (2-CHLORO-4-NITRO-PHENOXYMETHYL)-PYRIDINE (8G, 52percent) as a red solid. 2- (2-CHLORO-4-NITRO-PHENOXYMETHYL)-PYRIDINE (8 g, 30. 2MMOL, 1 eq) and 8. 44g iron (151.1 mmol, 5 eq) were mixed in 100 mL acetic acid and 50 mL ethyl acetate and were stirred at rt overnight. The reaction mixture was filtered through celite pad. The filtrate was concentrated in vacuo and neutralized with sat. NA2CO3 solution. The solution was extracted with ethyl acetate and the organic layer was washed with brine and concentrated in vacuo. The resulting crude material was purified by flash chromatography eluting with 30percent ethyl acetate/hexane yielding 3. 2G of 3-Chloro-4- (pyridin-2-ylmethoxy)-phenylamine as a white solid (52percent). 1H-NMR (CDCL3) No. 5.18 (s, 2H), 6.50 (dd, 1H), 6.76 (d, 1H),. 6.80 (d, 1H), 7.22 (m, 1 H), 7.64 (d, 1H), 7.73 (td, 1H), 8.55 (m, 1H) ; LCMS RT = 0.89 min; [M+H]+= 235.1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping