| 100% |
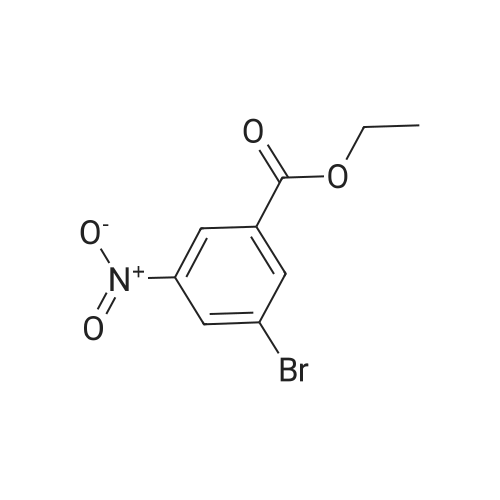
With caesium carbonate;bis-triphenylphosphine-palladium(II) chloride; In N,N-dimethyl-formamide; at 80℃; |
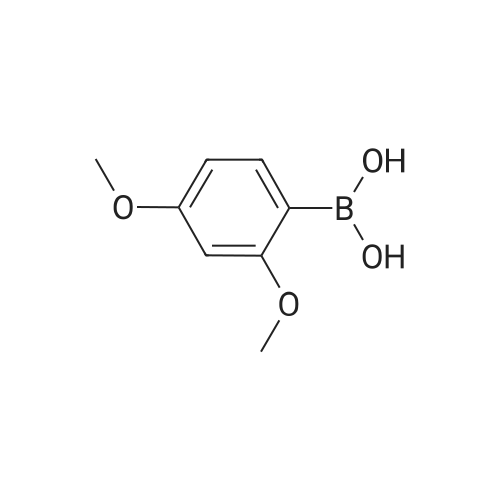
Methyl 2-(2,4-dimethoxyphenyl)-5-nitrobenzoate (Reference Compound No.1-1-(1)) A mixture of <strong>[133730-34-4]2,4-dimethoxyphenylboronic acid</strong> (25.0 g, 137 mmol), methyl 2-bromo-5-nitrobenzoate (35.7 g, 137 mmol), cesium carbonate (89.4 g, 274 mmol) and bis(triphenylphosphine)palladium (II) dichloride (4.81 g, 6.85 mmol) was suspended in N,N-dimethylformamide (450 mL), and then the suspension was stirred under argon atmosphere at 80C overnight. After cooling down, ethyl acetate (200 mL), diethylether (400 mL) and water (1000 mL) were added thereto and the mixture was separated into a water phase and an organic layer. The water layer was extracted with a mixed solvent of ethyl acetate (150 mL) - diethylether (150 mL) (twice). The combined organic layer was washed with water (500 mL, 3 times) and saturated brine (500 mL) successively, dried over anhydrous magnesium sulfate, and then the solvent was removed under reduced pressure to give the titled reference compound as a brown oil. (Quantitative) |
| 100% |
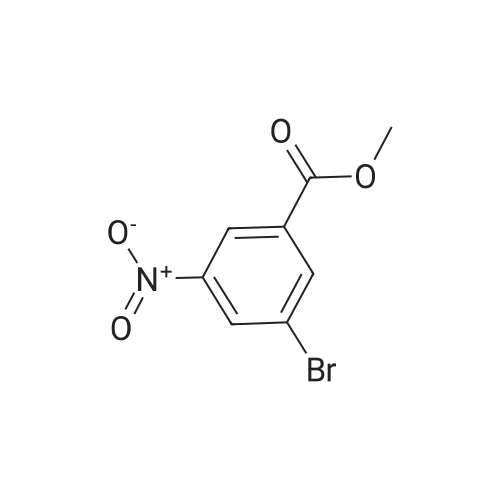
With caesium carbonate;bis-triphenylphosphine-palladium(II) chloride; In N,N-dimethyl-formamide; at 80℃;Inert atmosphere; |
Reference Example 1; 5-Hydroxymethyl-6-(2-methoxy-4-methoxymethoxyphenyl)-2,2,4-trimethyl-1,2-dihydroquinoline (Reference Compound No. 1)Methyl 2-(2,4-dimethoxyphenyl)-5-nitrobenzoate (Reference Compound No. 1-(1))A mixture of <strong>[133730-34-4]2,4-dimethoxyphenylboronic acid</strong> (25.0 g, 137 mmol), methyl 2-bromo-5-nitrobenzoate (35.7 g, 137 mmol), cesium carbonate (89.4 g, 274 mmol) and bis(triphenylphosphine)palladium (II) dichloride (4.81 g, 6.85 mmol) was suspended in N,N-dimethylformamide (450 mL), and then the suspension was stirred under argon atmosphere at 80 C. overnight. After cooling down, ethyl acetate (200 mL), diethylether (400 mL) and water (1000 mL) were added thereto and the mixture was separated into a water phase and an organic layer. The water layer was extracted with a mixed solvent of ethyl acetate (150 mL)-diethylether (150 mL) (twice). The combined organic layer was washed with water (500 mL, 3 times) and saturated brine (500 mL) successively, dried over anhydrous magnesium sulfate, and then the solvent was removed under reduced pressure to give the titled reference compound as a brown oil. (Quantitative) |
| 40% |
With tetrabutylammomium bromide; palladium diacetate; sodium carbonate; In water; at 150℃; for 0.166667h;Microwave irradiation; |
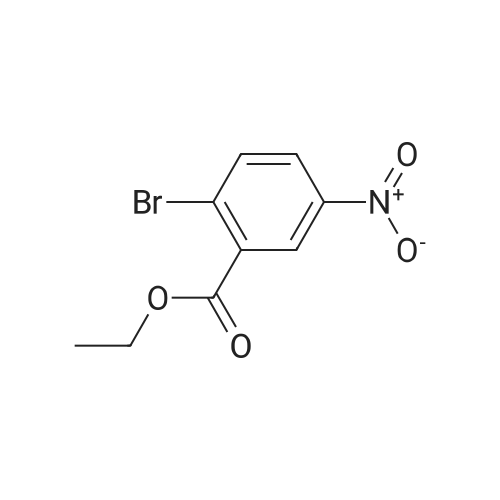
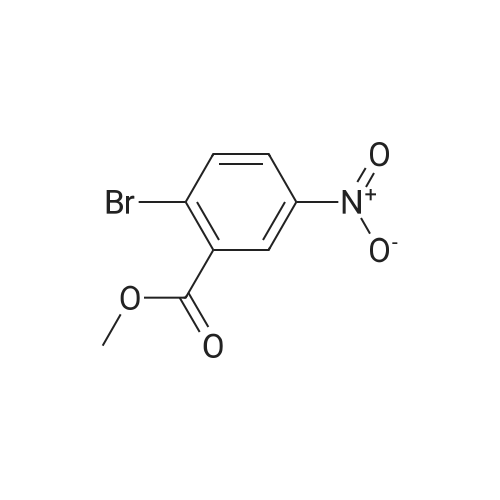
Commercially available <strong>[133730-34-4]2,4-dimethoxyphenylboronic acid</strong> (0.182 g, 1.00 mmol), sodium carbonate (0.318 g, 3.00 mmol), aryl bromide 4 (0.261 g, 1.00 mmol), palladium (II) acetate (0.0009 g, 0.004 mmol), tetra-n-butylammonium bromide (0.322 g, 1.00 mmol) and 10 mL of water were added to a microwave vial. The sealed vial was heated in the microwave for 10 min at 150 C. The reaction was then allowed to cool to room temperature, diluted with 100 mL of water, and extracted 2x with ether. Combined ether extracts were then washed with brine and dried with MgSO4. The solvents were removed under reduced pressure. A yellow oil was isolated by column chromatography (0.6318 g, 40%). 1H NMR (400 MHz, CDCl3): δ 8.67 (d, J=2.2 Hz, 1 H), 8.34 (dd, J=8.3, 2.5 Hz, 1 H), 7.49 (d, J=8.5 Hz, 1 H), 7.20 (d, J=8.5 Hz, 1 H), 6.61 (dd, J=8.4, 2.4 Hz, 1 H), 6.49 (d, J=2.2 Hz, 1 H), 3.87 (s, 3 H), 3.76 (s, 3 H), 3.71 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 166.85, 161.67, 156.92, 146.31, 145.16, 132.90, 132.51, 130.41, 125.82, 124.67, 121.06, 104.82, 98.42, 55.45, 55.18, 52.26. FT-IR (thin film, cm-1): 1734.34, 1609.70, 1523.04, 1349.12. TOF-MS (ESI, [M+H]+) calculated for C16H16NO6 318.0972; found 318.0952 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping