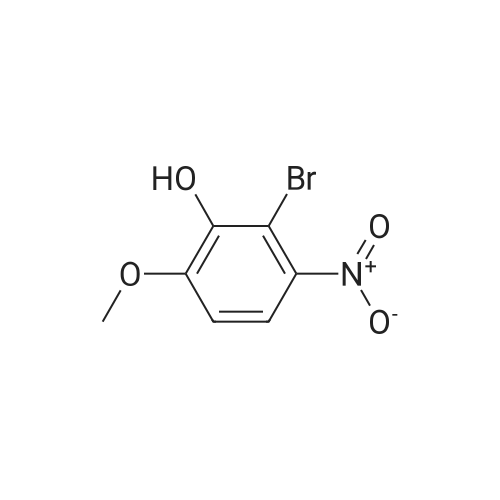
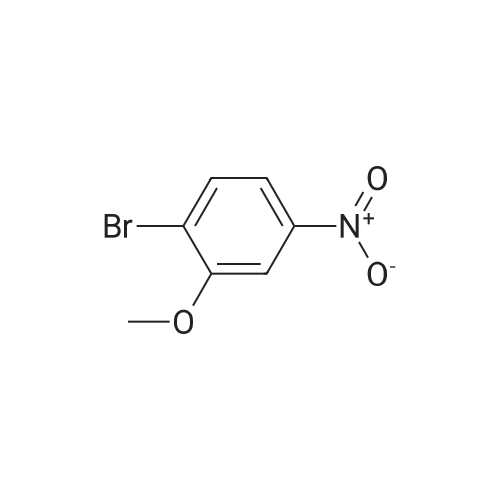
| 95% |
With iron; ammonium chloride; In tetrahydrofuran; methanol; water; for 1h;Reflux; |
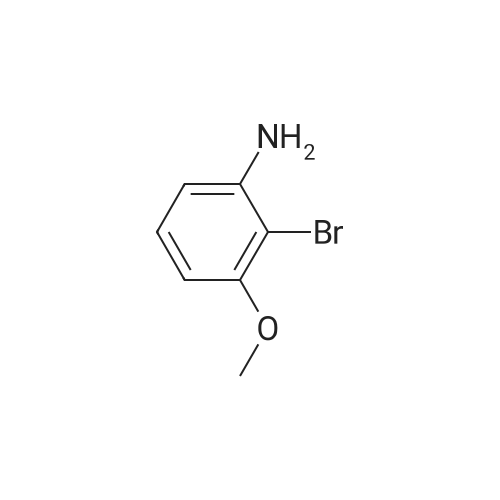
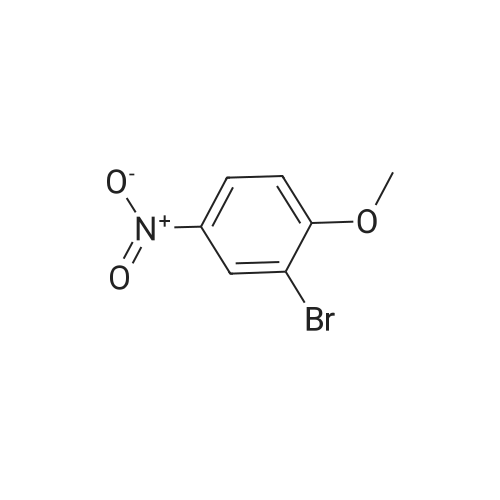
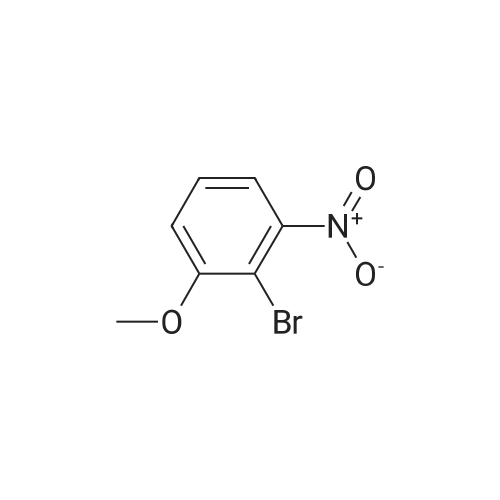
2-bromo-1-methoxy-3-nitrobenzene (1 g, 4.31 mmol), Fe (1.68 g, 30.17 mmol) and NH4Cl (1.61 g, 30.17 mmol)were dissolved in THF (4 mL)/MeOH (4 mL)/H2O (2 mL) solution and stirred for 1 hour under reflux. After terminationof the reaction, the reaction solution was cooled to room temperature, diluted with saturated NaHCO3 solution andextracted with EtOAc. The extract solution was concentrated under reduced pressure and purified by column chromatography(eluent, EtOAc/Hex = 1/5) to obtain the title compound (0.83 g, 95 % yield).1H NMR (500 MHz, CDCl3) delta 7.05(dd, 1H), 6.42(d, 1H), 6.31(d, 1H), 3.86(s, 3H) |
| 91% |
With acetic acid;iron; In ethanol; for 3.5h;Heating / reflux; |
2-Bromo-3-nitroanisole B3 (1.00 g; 4.31 MMOL) was dissolved in glacial acetic acid (11.0 mL) and ethanol (11.0 mL). To this solution was added iron powder (0.98 g; 17.5 MMOL). The mixture was stirred at reflux for 3.5 h and worked up. The reaction mixture was diluted with water (35 mL), neutralized with solid Na2CO3 and the product extracted with CH2CI2 (3X 50 mL). The extracts were dried (NA2SO4), filtered and CONCENTRATED IN VACUO to afford the crude product, 2-bromo-3 methoxyaniline B4 (91 %; 0. 79 g) as a pale yellow oil. MS 201.8 (MH) + ; Homogeneity by HPLC (TFA) 220 nm: 95%. |
| 91% |
With iron; acetic acid; In ethanol; for 3.5h;Heating / reflux; |
Step C:; 2-Bromo-3-nitroanisole 1b3 (1.00 g; 4.31 mmol) was dissolved in glacialacetic acid (11.0 mL)/ethanol (11.0 mL) and to the solution was added iron powder(0.98 g; 17.5 mmol). The mixture was stirred at reflux for 3.5 hr and worked up. Thereaction mixture was diluted with water (35 mL), neutralized with solid Na2CO3 and theproduct extracted with CH2CI2 (3X 50 mL). The extracts were dried (Na2SO4), filteredand concentrated in vacua to afford the crude product, 2-bromo-3 methoxyaniline 1b4(91%; 0.79 g) as a pale yellow oil. MS 201.8 (MH)+; Homogeneity by HPLC (TFA) (at)220nm: 95% |
| 91% |
With iron; acetic acid; In ethanol; for 3.5h;Heating / reflux; |
2-Bromo-3-nitroanisole 1b3 (1.00 g; 4.31 mmol) was dissolved in glacial acetic acid (11.0 mL)/ethanol (11.0 mL) and to the solution was added iron powder (0.98 g; 17.5 mmol). The mixture was stirred at reflux for 3.5 hr and worked up. The reaction mixture was diluted with water (35 mL), neutralized with solid Na2CO3 and the product extracted with CH2Cl2(3*50 mL). The extracts were dried (Na2SO4), filtered and concentrated in vacuo to afford the crude product, 2-bromo-3 methoxyaniline 1b4 (91%; 0.79 g) as a pale yellow oil. MS 201.8 (MH)+; Homogeneity by HPLC (TFA)a220 nm: 95% |
| 91% |
With iron; acetic acid; In ethanol; for 3.5h;Heating / reflux; |
2-Bromo-3-nitroanisole 2b3 (1.00 g; 4.31 mmol) was dissolved in glacialacetic acid (11.0 mL )/ethanol (11.0 mL) and to the solution was added iron powder(0.98 g; 17.5 mmol). The mixture was stirred at reflux for 3.5 hr and worked up. Thereaction mixture was diluted with water (35 mL), neutralized with solid Na2CO3 andthe product extracted with CH2CI2( 3X 50 mL). The extracts were dried (Na2SO4),filtered and concentrated in vacuo to afford the crude product, 2-bromo-3methoxyaniline 2b4 (91%; 0.79 g) as a pale yellow oil. MS 201.8 (MH)+;Homogeneity by HPLC (TFA) (at) 220nm: 95% |
| 91% |
|
Step C; 2-Bromo-3-nitroanisole 2B3 (1.00 g; 4.31 mmol) was dissolved in glacial acetic acid (11.0 mL)/ethanol (11.0 mL) and to the solution was added iron powder (0.98 g; 17.5 mmol). The mixture was stirred at reflux for 3.5 h and worked up. The reaction mixture was diluted with water (35 mL), neutralized with solid Na2CO3 and the product extracted with CH2CI2 (3X 50 mL). The extracts were dried (Na2SO4), filtered and concentrated in vacuo to afford the crude product, 2-bromo-3 methoxyaniline 2B4 (91%; 0.79 g) as a pale yellow oil. MS 201.8 (MH)+; Homogeneity by HPLC (TFA) (at) 220nm: 95% |
| 89% |
With water; iron; ammonium chloride; In tetrahydrofuran; at 70℃; for 2.5h; |
A solution of 2-bromo-l-methoxy-3 -nitrobenzene (500 mg, 2.16 mmol) in CH3OH (5 mL) and THF (5 mL) was added to a solution OfNH4Cl (572 mg, 10.7 mmol) in water (5 mL). Then, iron (325 mesh, 601 mg, 10.7 mmol) was added and the resulting mixture was heated to 70 C under nitrogen. After 2.5 h, the reaction mixture was cooled to room temperature, filtered over kieselguhr, diluted with AcOEt, and successively washed with a saturated solution OfNaHCO3 in water and brine. The organic layer was dried (Na2SO4) and evaporated. The residue was triturated in CH2Cl2, the filtered to give 390 mg (89 %) of the target product 37 as a beige solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping