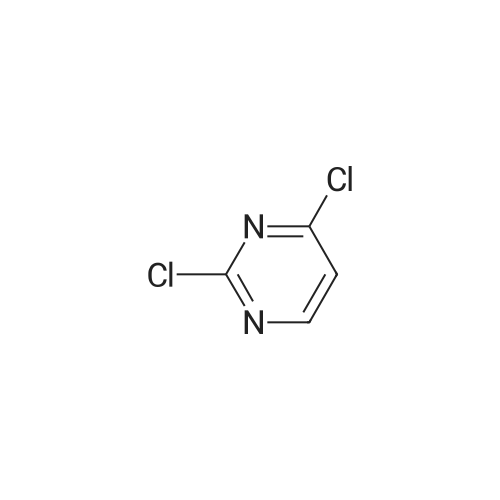
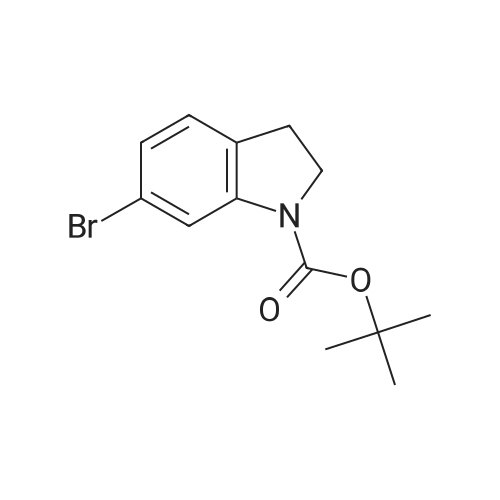
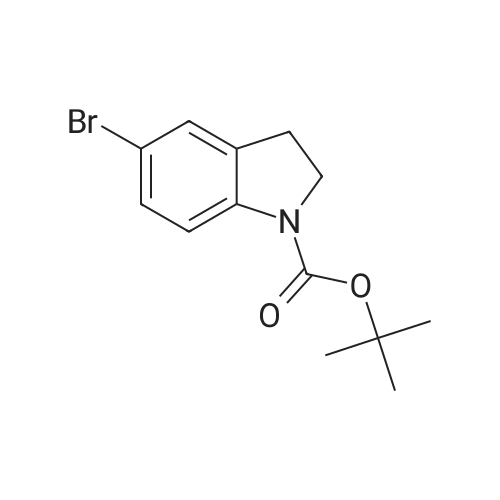
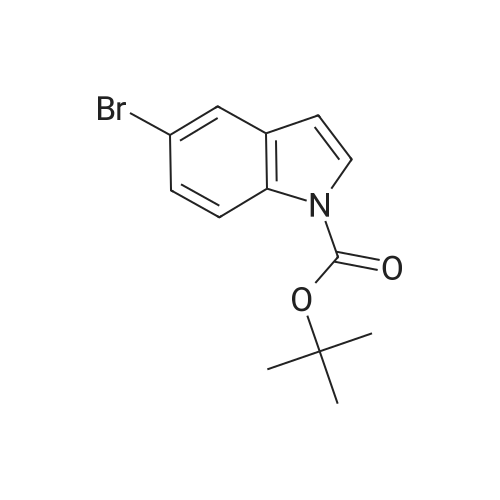
| 85% |
With dmap; triethylamine; In acetonitrile; at 20℃; for 2h; |
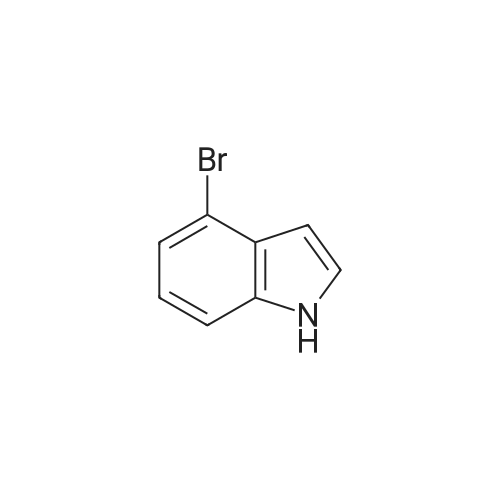
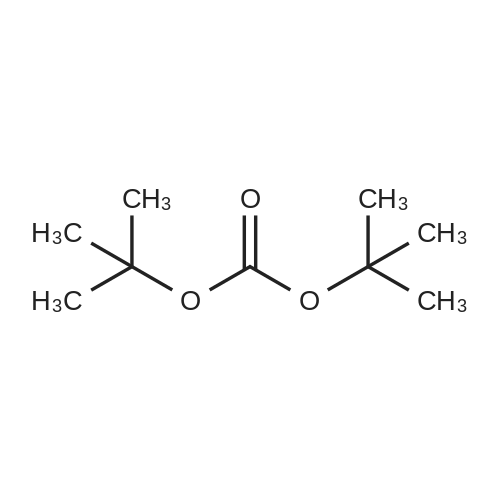
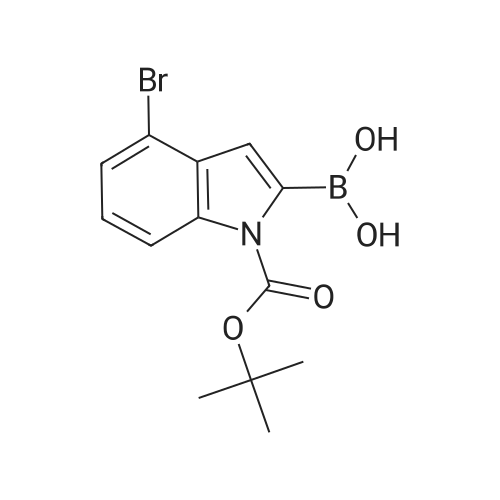
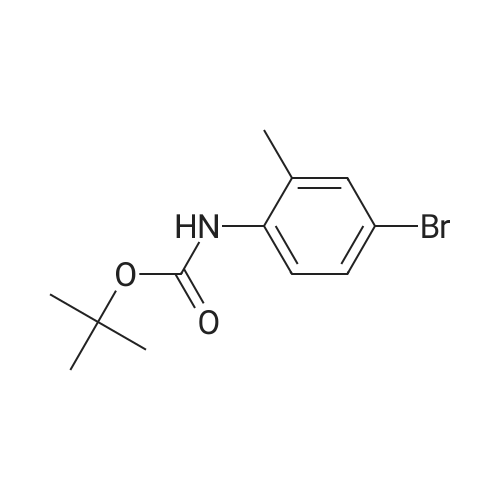
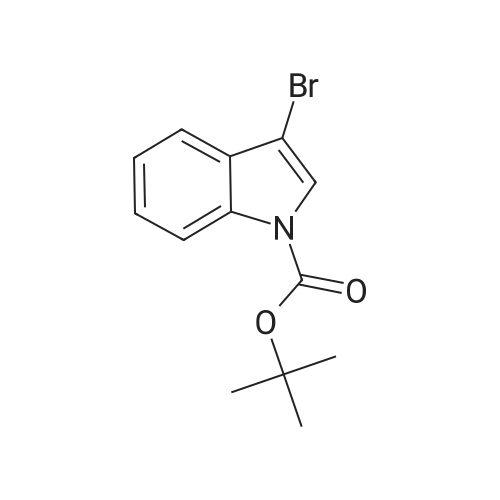
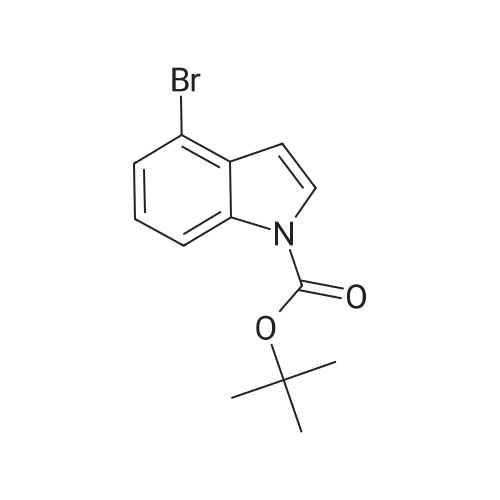
Step 84a: 7?rt-butyl 4-bromo-lH-indole-l-carboxylate (Compound 0601-176)The solution of 4-bromoindole (394 mg, 2.00 mmol), (Boc)20 (523 mg, 2.40 mmol), DMAP (293 mg, 2.4 mmol) and Et3N (0.4 mL) in MeCN (6 mL) was stirred at room temperature for 2 hours. The solvent was removed and the residue was dissolved in ethyl acetate (40 mL), washed with water (3 x 20 mL) and brine (1 x 20 mL), the organic layer was concentrated and purified by column chromatography on silica gel (petroleum ether) to afford compound 0601-176 (508 mg, 85%) as a colorless oil. 1H-NMR (400 MHz.OMSO-d6) delta 1.64 (s, 9H), 6.67 (d, J= 3.2 Hz, 1H), 7.28 (t, J= 8.0 Hz, 1H), 7.48 (m, 1H),7.80 (d, J= 3.2 Hz, 1H), 8.08 (m, 1H). |
| 85% |
With dmap; triethylamine; In acetonitrile; at 20℃; for 2h; |
Step 84a: Tert-butyl 4-bromo-1H-indole-1-carboxylate (Compound 0601-176)[0559]The solution of 4-bromoindole (394 mg, 2.00 mmol), (Boc)2O (523 mg, 2.40 mmol), DMAP (293 mg, 2.4 mmol) and Et3N (0.4 mL) in MeCN (6 mL) was stirred at room temperature for 2 hours. The solvent was removed and the residue was dissolved in ethyl acetate (40 mL), washed with water (3×20 mL) and brine (1×20 mL), the organic layer was concentrated and purified by column chromatography on silica gel (petroleum ether) to afford compound 0601-176 (508 mg, 85%) as a colorless oil. 1H-NMR (400 MHz. DMSO-d6) delta 1.64 (s, 9H), 6.67 (d, J=3.2 Hz, 1H), 7.28 (t, J=8.0 Hz, 1H), 7.48 (m, 1H), 7.80 (d, J=3.2 Hz, 1H), 8.08 (m, 1H). |
| 85% |
With dmap; triethylamine; In acetonitrile; at 20℃; for 2h; |
MeCN (6 mL) solution of 4-bromo-indole (394mg, 2.00mmol), (Boc) 2O (523mg, 2.40mmol), was DMAP (293mg, 2.4mmol) to a solution of and Et3N (0.4mL) was stirred at room temperature for 2 hours . The solvent was removed and the residue was dissolved in ethyl acetate (40 mL), washed with water (3 × 20 mL) and brine (1X20mL), and the organic layer concentrated, and purified by column chromatography on silica gel (petroleum ether) on, to give compound 0601-176 as a colorless oil (508mg, 85%). |
| 76% |
With dmap; triethylamine; In dichloromethane; at 0 - 20℃; for 2h; |
To a solution of 4-bromo-lH-indole (LXXXVII) (10 g, 50.8 mmol, 1 eq), DMAP (622 mg, 5.1 mmol, 0.1 eq) and TEA (10.6 ml, 76.1 mmol, 3 eq) in DCM (200 mL) was added B0C2O (14.4 mL, 61 mmol, 1.2 eq) at 0C. The reaction was warmed to room temperature and stirred for 2 h. Water (200 mL) was added and the mixture was extracted with DCM twice. The solvent was evaporated under vacuum to give fert-butyl 4-bromo-lH-indole-l-carboxylate (LXXXVIII) as white solid (11.4 g, 38.5 mmol, 76% yield). NMR (CDC13, 400 MHz) delta ppm 1.68 (s, 9H), 6.64 (d, J=4Hz, 1H), 7.17 (t, J=8.4Hz, 1H), 7.39 (d, J=7.6Hz, 1H), 7.64 (d, J=3.2Hz, 1H), 8.11 (d, J=8.0Hz, 1H); ESIMS found for Ci3Hi4BrN02 mlz 297.1 (M+H). |
| 76% |
With dmap; triethylamine; In dichloromethane; at 0 - 20℃; for 2h; |
To a solution of 4-bromo-1H-indole (LXX) (10 g, 50.8 mmol, 1 eq), DMAP (622 mg, 5.1 mmol, 0.1 eq) and TEA (10.6 ml, 76.1 mmol, 3 eq) in DCM (200 mL) was added Boc2O (14.4 mL, 61 mmol, 1.2 eq) at 0C. The reaction was warmed to room temperature and stirred for 2 h. Water (200 mL) was added and the mixture was extracted with DCM twice. The solvent was evaporated under vacuum to give tert-butyl 4-bromo-1H-indole-1-carboxylate (LXXI) as white solid (11.4 g, 38.5 mmol, 76% yield). 1H NMR (CDCI3, 400 MHz) delta ppm 1.68 (s, 9H), 6.64 (d, J=4Hz, 1H), 7.17 (t, J=8.4Hz, 1H), 7.39 (d, J=7.6Hz, 1H), 7.64 (d, J=3.2Hz, 1H), 8.11 (d, J=8.0Hz, 1H); ESIMS found for C13H14BrNO2 m/z 297.1 (M+H). |
| 76% |
With dmap; triethylamine; In dichloromethane; at 0 - 20℃; for 2h; |
To a solution of 4-bromo-1H-indole (LXX) (10 g, 50.8 mmol, 1 eq), DMAP (622 mg, 5.1 mmol, 0.1 eq) and TEA (10.6 ml, 76.1 mmol, 3 eq) in DCM (200 mL) was added Boc2O (14.4 mL, 61 mmol, 1.2 eq) at 0C. The reaction was warmed to room temperature and stirred for 2 h. Water (200 mL) was added and the mixture was extracted with DCM twice. The solvent was evaporated under vacuum to give tert-butyl 4-bromo- 1H-indole- 1 -carboxylate (LXXI) as white solid (11.4 g, 38.5 mmol, 76% yield). ?HNMR(CDC13, 400 MHz) Eppm 1.68 (s, 9H), 6.64 (d,J=4Hz, 1H), 7.17 (t,J=8.4Hz, 1H), 7.39 (d,J=7.6Hz, 1H), 7.64 (d,J=3.2Hz, 1H), 8.11 (d, J8.OHz, 1H); ESIMS found for C,3H,4BrNO2 mlz 297.1 (M+H). |
| 66.19% |
|
To a suspension of 60% NaH (0.449 g, 11.2 mmol) in dry THF (20.0 mL) was added a solution of compound 1 (2.000 g, 10.20 mmol) in THF (20.0 mL) at -78 C. The reaction mixture was stirred for 1 h. A solution of ditertiary butyl dicarbonate (2.58 mL, 11.2 mmol) in THF (20.0 mL) was added to the above solution drop-wise at -78 C. and stirred at rt for 16 h The reaction progress was monitored by diluting an aliquot of the reaction mixture with water and extracting with EtOAc. The organic layer was spotted over an analytical silica gel TLC plate and visualized using 254 nm UV light. The reaction progressed to completion with the formation of a non-polar spot. The Rf values of the starting material and product were 0.3 and 0.5, respectively. The reaction mixture was poured into ice water (75.0 mL) and extracted with EtOAc (2×100.0 mL). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to afford crude compound 2. The crude compound was purified by flash column using 230-400 mesh silica gel and eluted with 8% EtOAc in petroleum ether to afford compound 2 as a brown liquid. TLC system: 5% EtOAc in petroleum ether. Yield 2.000 g (66.19%). |
|
With dmap; triethylamine; In dichloromethane; at 0 - 20℃; for 2h; |
To a solution of 4-bromo-lH-indole (LXX) (10 g, 50.8 mmol, 1 eq), DMAP (622 mg, 5.1 mmol, 0.1 eq) and TEA (10.6 ml, 76.1 mmol, 3 eq) in DCM (200 mL) was added B0C2O (14.4 mL, 61 mmol, 1.2 eq) at 0C. The reaction was warmed to room temperature and stirred for 2 hours. Water (200 mL) was added and the mixture was extracted with DCM twice. The solvent was evaporated under vacuum to give fert-butyl 4-bromo-lH-indole-l-carboxylate (LXXI) as white solid (11.4 g, 38.5 mmol, 76% yield). NMR (CDC13, 400 MHz) delta ppm 1.68 (s, 9H), 6.64 (d, J=4Hz, 1H), 7.17 (t, J=8.4Hz, 1H), 7.39 (d, J=7.6Hz, 1H), 7.64 (d, J=3.2Hz, 1H), 8.11 (d, J=8.0Hz, 1H); ESIMS found for Ci3Hi4BrN02 mlz 297.1 (M+H). |
|
With dmap; In tetrahydrofuran; for 0.5h; |
Di-tert-butyl dicarbonate (5.33 mL, 22.95 mmol) was added to a solution of 4-bromo-1H-indole (3.00 g, 15.30 mmol) and DMAP (4-dimethylaminopyridine) (0.187 g, 1.530 mmol)) in tetrahydrofuran (20 mL). The reaction mixture was stirred for 30 minutes. The mixture was partitioned between ether (100 mL) and water (100 mL). The ether layer was washed with (3*50 mL) of saturated aqueous sodium bicarbonate and 75 mL of brine. The ether layer was dried over magnesium sulfate, filtered and concentrated. The residue was chromatographed over silica gel eluting with 1:100 ethyl acetate: petroleum ether to afford the title compound. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping