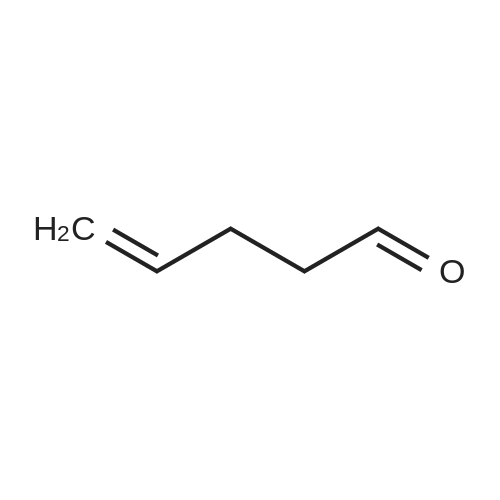
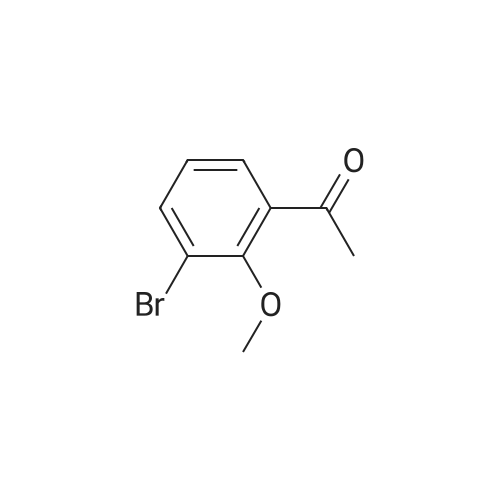
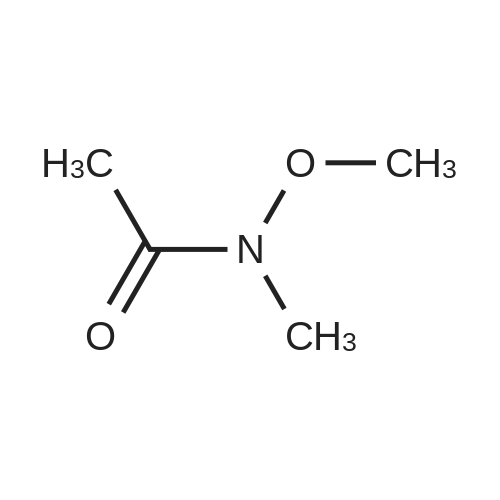
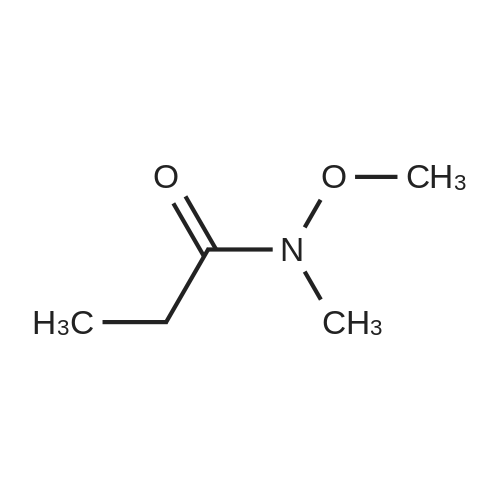
| 98% |
In acetonitrile; at 80℃; for 20h; |
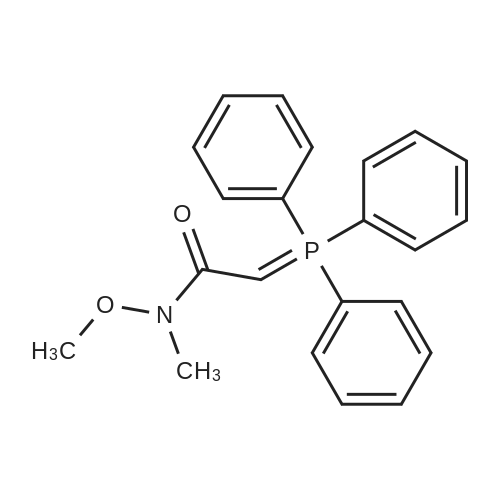
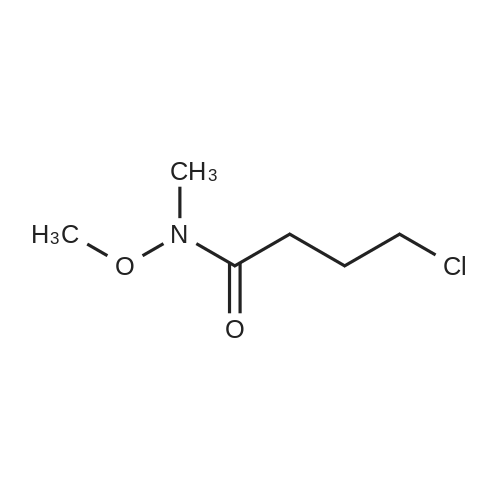
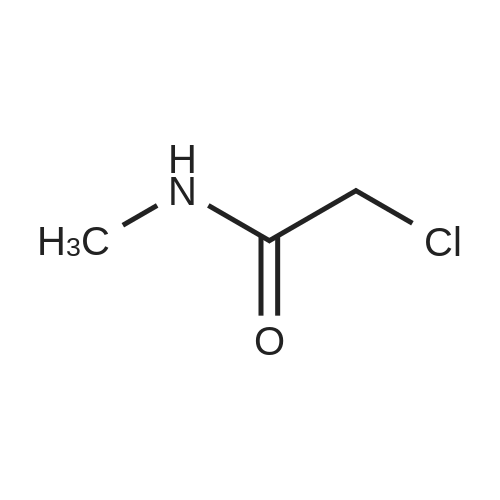
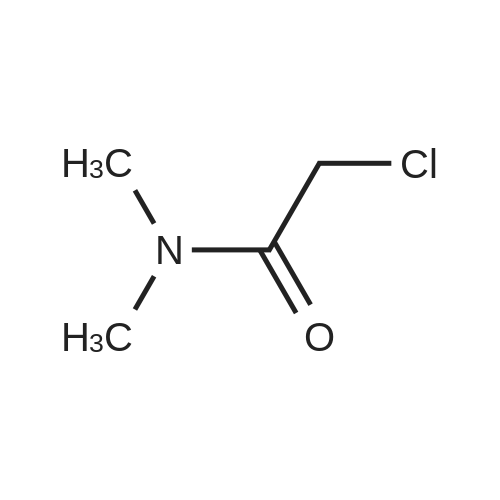
Step 1: N-Methoxy-N-methyl-2-(triphenyl-15-phosphanylidene)acetamide A mixture of (<strong>[67442-07-3]2-chloro-N-methoxy-N-methylacetamide</strong> (13.7 g, 0.1 mol) and triphenylphosphane (26.2 g, 0.1 mol) in acetonitrile (200 mL) was heated to 80° C. and held for 20 h. The mixture was cooled and concentrated to remove the solvent below 40° C. The residue was dissolved in dichloromethane (200 mL), followed by 2 N KOH (100 mL). The resulting mixture was stirred at 20° C. for 1 h. Phase separation, the organic layer was washed with brine (200 mL*3), dried over Na2SO4 and filtered. The filtrate was concentrated in vacuum to afford N-methoxy-N-methyl-2-(triphenyl-15-phosphanylidene) acetamide (36 g, 0.1 mol, 98percent) as a yellow solid. ESI-MS (EI+, m/z): 364.4 [M+H]+. |
| 98% |
In acetonitrile; at 80℃; for 20h; |
A mixture of (<strong>[67442-07-3]2-chloro-N-methoxy-N-methylacetamide</strong> (13.7 g, 0.1 mol) and triphenylphosphane (26.2 g, 0.1 mol) in acetonitrile (200 mL) was heated to 80 °C and held for 20 h. The mixture was cooled and concentrated to remove the solvent below 40 °C. The residue was dissolved in dichloromethane (200 mL), followed by 2 N KOH (100 mL). The resulting mixture was stirred at 20 °C for lh. Phase separation, the organic layer was washed with brine (200 mL*3), dried over Na2SO4 and filtered. The filtrate was concentrated in vacuum to afford N-methoxy-N-methyl-2-(triphenyl-15-phosphanylidene) acetamide (36 g, 0.1 mol, 98percent) as a yellow solid. ESI-MS (EI, m/z): 364.4 [M+H] . |
|
In acetonitrile; for 18h;Heating / reflux; |
Synthesis of ylide 21. To a solution of N-methoxy-N-methylhydroxylamine hydrochloride (48.52 g, 500 mmol, 1.0 equiv) in CH2Cl2 (1.0 L) at 0°C were added pyridine (80.63 mL, 1.0 mol) and chloroacetic anhydride (85.14 g, 500 mmol, 1.0 equiv). The resulting mixture was stirred for 15 min at 00C, then warm up to 23°C and stirred overnight. The reaction mixture was then poured carefully into sat. NaHCpsi3aq solution (1.0 L) and stirred 1 hour, after which the layers were separated, the aqueous phase was extracted with CHaCl2 (400 mL) and the combined organic layers were washed with IN HCl (200 mL x 2), brine (200 mL x 2), dried over Na2SO4 (10 g) and filtered. Evaporation of the solvents under reduced pressure afforded the corresponding acetamide (N-rnethoxy-N-methyl acetamide-2-chloride) as a green oil which was used in the next step without further purification. To a solution of this acetamide in CH3CN (800 mL) was added Ph3P (107.98 g, 411.7 mmol, 0.82 equiv) and the resulting mixture was refluxed for 18 hours. Then the solvents were removed under vacuum and the resulting viscous oil was dissolved in CH2Cl2 (1.0 L), washed with 2N KOH (400 mL x 2), brine (400 mL) and dried over Na2SO4 (10.0 g). Filtration and evaporation of the solvents under reduced pressure afforded ylide 21 as a thick oil which solidified by standing (146.5 g, 80percent over two steps). This compound was used in the next step without further purification. Rf = 0.85 (Hexane/EtOAc 3/1); 1H NMR (CDCl3, 400 MHz, 25 0C) 57.71-7.65 (m, 6H), 7.55-7.50 (m, 3H), 7.48-7.42 (m, 6H), 3.74 (s, 3H), 3.08 (s, 3H), 1.86 (s, IH); 13C NMR <n="150"/>(CDCl3, 100 MHz, 25 0C) 133.3 (x 3), 133,2 (x 3), 131.9 (x 3), 128.9 (x 3), 128.8 (x 3), 127.9, 61.3, 35.9. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping