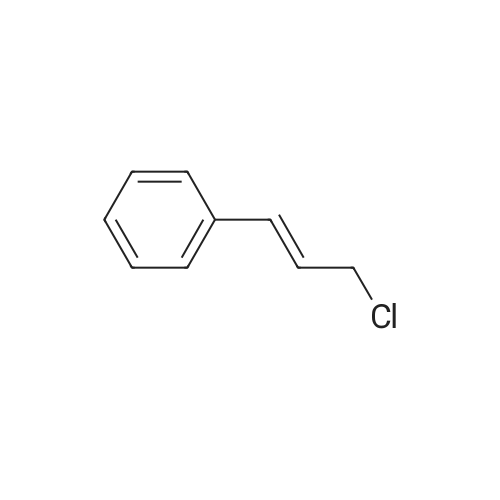
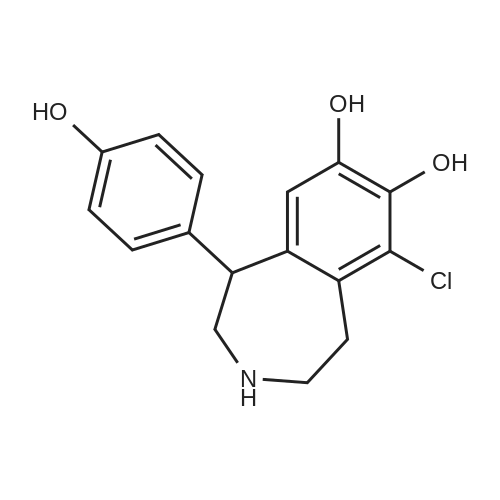
|
With methanesulfonic acid; water; In methanol;pH 2.3 - 3.7;Product distribution / selectivity; |
Example 1 - Preparation of Fenoldopam Mesylate Type I; [00056] 200 grams of <strong>[67227-57-0]fenoldopam mesylate</strong> were obtained and purified by column chromatography, followed by a single crystallization. The wet crystals of <strong>[67227-57-0]fenoldopam mesylate</strong> were then combined with a mixture of 560 grams of methanol and 1400 grams of water and 0.2 g of methanesulfonic acid, sufficient to provide a pH EPO <DP n="15"/>of 3.7 to form a solution, which was cla?fied through a disposable filter unit, and concentrated under vacuum in an evaporating flask with an external bath temperature of 600C to about 950 grams. The suspension was then cooled at 0 to 20C with stirring overnight, and then filtered. The filter cake was washed with 100 grams of cold water, and the product was dried in an oven at 80C under vacuum for 16 hours, providing a yield of 172.4 grams of product. An XRD analysis confirmed the sample was Type I.Example 12 - Preparation of Fenoldopam Mesylate Type I; A solution of 300 grams of <strong>[67227-57-0]fenoldopam mesylate</strong> (after chromatography) in a mixture of 2420 grams of methanol and 17740 grams of water and 11 grams of methanesulfonic acid, sufficient to provide a pH of 2.3, was clarified through a disposable filter cartridge, rinsed with 200 grams of methanol, and concentrated under vacuum in an evaporating flask at an external bath temperature of 700C to about 1800 grams. The suspension was cooled at room temperature with stirring, left overnight, and then cooled at 0 to 20C for 2 hours, and filtered. The filter cake was washed with 210 grams of water, and the wet crystals were washed with 750 grams of isopropanol. An XRD analysis confirmed the sample was <strong>[67227-57-0]fenoldopam mesylate</strong> Type I.Example 13 - Preparation of Fenoldopam Mesylate Type I; [00067] A solution of 300 grams of <strong>[67227-57-0]fenoldopam mesylate</strong> (after chromatography) in a mixture of 2420 grams of methanol and 17740 grams of water and 11 grams of methanesulfonic acid, having a pH of 2.3, was clarified through a disposable filter cartridge, rinsed with 200 grams of methanol, and concentrated under vacuum in an evaporating flask at an external bath temperature of 70C to about 1800 grams. The suspension was cooled at room temperature with stirring, left overnight, then cooled at 0 to 2 for 2 hours, and filtered. The filter cake was washed with 210 grams of water, and an XRD analysis confirmed the sample was <strong>[67227-57-0]fenoldopam mesylate</strong> Type . |
|
With methanesulfonic acid; water;pH 2.2;Product distribution / selectivity; |
Example 6 - Preparation of Fenoldopam Mesylate Type I; [00061] A 16 gram sample of <strong>[67227-57-0]fenoldopam mesylate</strong> was dissolved in 800 grams of water. The pH of the resulting solution was adjusted to 2.2 with methanesulfonic acid, the solution was concentrated under vacuum to 160 grams, and cooled with stirring at room temperature. The resulting suspension was then left to crystallize in a refrigerator, without stirring, at a temperature of from 0 to 5 overnight. The suspension was then filtered, and a small sample of this wet cake was analyzed by XRD, which demonstrated that the sample was <strong>[67227-57-0]fenoldopam mesylate</strong> Type I. |
|
With water; In methanol;Product distribution / selectivity; |
Example 2 - Preparation of Fenoldopam Mesylate Type I; [00057] 200 grams of <strong>[67227-57-0]fenoldopam mesylate</strong> were obtained and purified by column chromatography, followed by a single crystallization. The wet crystals of <strong>[67227-57-0]fenoldopam mesylate</strong> were then combined with a mixture of 560 grams of methanol and 1400 grams of water to form a solution, which was clarified through a disposable filter unit, and concentrated under vacuum in an evaporating flask with an external bath temperature of 60C to about 950 grams. The suspension was then cooled at 0 to 20C with stirring for 2 hours, and then filtered. The filter cake was washed with 100 grams of cold water, and the product was dried in an oven at 800C under vacuum for 16 hours, providing a yield of product of 167.6 grams, containing 0.4 percent by weight water. An XRD analysis confirmed the sample was <strong>[67227-57-0]fenoldopam mesylate</strong> Type I. Example 3 - Preparation of Fenoldopam Mesylate Type I; [00058] 165 grams of <strong>[67227-57-0]fenoldopam mesylate</strong> were obtained and purified by column chromatography, followed by a single crystallization. The wet crystals of <strong>[67227-57-0]fenoldopam mesylate</strong> were then combined with a mixture of 462 grams of methanol and 1155 grams of water to form a solution, which was clarified through a disposable filter unit, and concentrated under vacuum in an evaporating flask with an external bath temperature of 700C to about 770 grams. Then, 1000 grams of water were added, the suspension was evaporated to 770 grams, cooled at 0 to 2C with stirring for 2 hours, and then filtered. The filter cake was washed with 82 grams of cold water, and the product was dried in an oven at 80 C under vacuum for 16 hours, providing a product yield of 101.8 grams, having an initial water content of 0.94 percent by weight. An XRD analysis confirmed the sample was <strong>[67227-57-0]fenoldopam mesylate</strong> Type . EPO <DP n="16"/>S-kappa u i / '(J b> Ii i&/ -J +/- B Jr1 b _ _, , . _ _ ,Example 4 - Preparation of Fenoldopam Mesylate Type I; [00059] 50 grams of <strong>[67227-57-0]fenoldopam mesylate</strong> were obtained and purified by column chromatography, followed by a single crystallization. The wet crystals of <strong>[67227-57-0]fenoldopam mesylate</strong> were then combined with a mixture of 140 grams of methanol and 350 grams of water to form a solution, which was clarified through a disposable filter unit, and concentrated under vacuum in an evaporating flask, having an external bath temperature of 7O0C, to about 230 grams. The suspension was cooled at 0 to 20C with stirring for 2 hours, and then filtered. The filter cake was washed with 100 grams of cold water, and the product was dried in an oven at 80C under vacuum for 16 hours, providing a product yield of 38 grams, having an initial water content of 0.2 percent by weight. An XRD analysis confirmed the sample was <strong>[67227-57-0]fenoldopam mesylate</strong> Type . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science












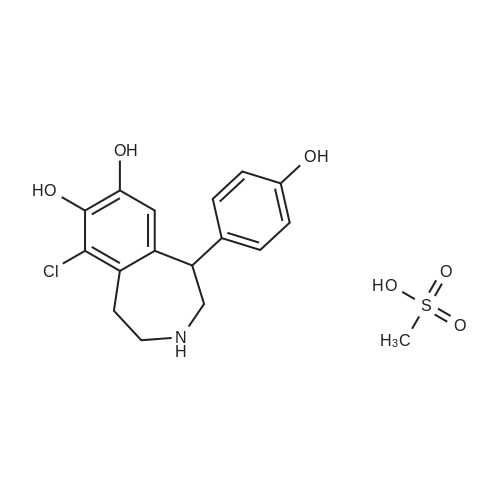

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping