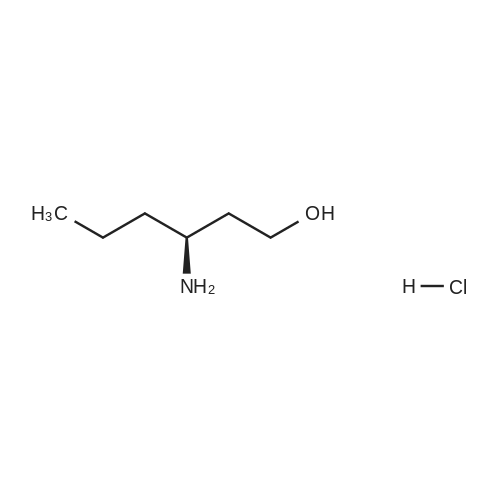
| 98% |
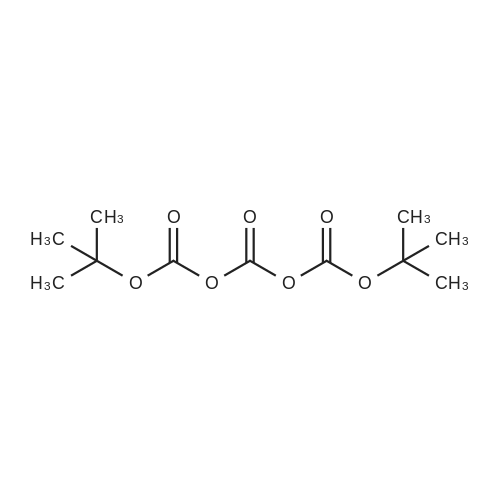
With sodium hydroxide; In water; tert-butyl alcohol; for 18h;Inert atmosphere; |
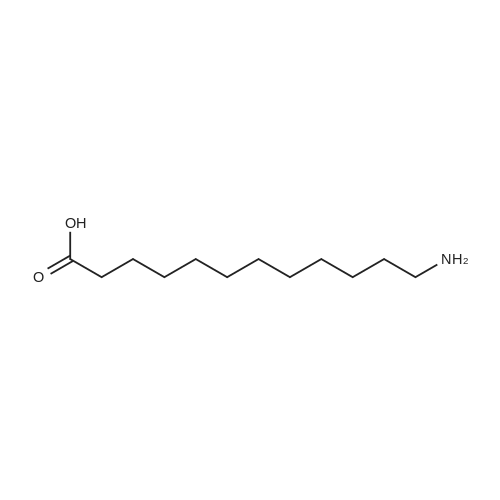
To a mixture of NaOH (43 mg, 1.1 mmol), in water/tert-BuOH 1:1 (1.0 mL) was added di-tert-butyl dicarbonate (225 muL, 1.05 mmol) and <strong>[67107-87-3]12-amino-dodecan-1-ol</strong> (199 mg, 1.00 mmol). After the viscous mixture was stirred for 18 h, 0.3 M HCl solution (1.5 mL) was added and the aqueous layer was extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (5 mL) and dried over MgSO4. The solvent was removed under reduced pressure and 294 mg (98%) of the product was obtained as white solid. Mp 75 C. 1H NMR (300 MHz, CDCl3) delta 4.51 (s, 1H, NH), 3.66 (dd, J = 11.1, 6.5 Hz, 2H, NHCH2), 3.11 (dd, J = 13.2, 6.6 Hz, 2H, CH2OH), 1.73-1.16 (m, 21H, (CH2)10, OH), 1.46 (s, 9H, OtBu). 13C NMR (151 MHz, CDCl3) delta 155.94, 78.96, 63.03, 40.60, 32.77, 30.03, 29.53, 29.48, 29.48, 29.37, 29.23, 28.40, 26.77, 25.69. IR (film) nu = 3368 cm-1, 2919, 2851, 1685, 1653, 1558, 1523, 1481, 1469, 1457, 1443, 1389, 1364, 1337, 1288, 1266, 1243, 1225, 1173, 1142, 1059, 1027, 994, 978, 867, 781, 741, 720, 679, 661. HRMS (ESI) calcd for C17H35NNaO3 [M+Na]+ 324.2515, found 324.2509. |
|
In tetrahydrofuran; at 50℃; for 3h;Inert atmosphere; |
General procedure: To a solution of an aminoalcohol (66.6 mmol) in THF (67 mL) was added di-tert-butyl dicarbonate (15.3 g, 66.6 mmol) at 50 C under an argon atmosphere. After 3 h stirring, the solvent was removed in vacuo. The crude product was purified by silica gel column chromatography (EtOAc / hexane, 50:50) to give a N-Boc aminoalcohol. Iodine (12.5 g, 49.3 mmol) was added to a solution of N-Boc aminoalcohol (17.6 mmol), PPh3 (6.92 g, 26.3 mmol) and imidazole (1.79 g, 26.3 mmol) in CH2Cl2 (98 mL) at 0 C. The mixture was stirred at room temperature for 1 h under an argon atmosphere. The reaction mixture was quenched with saturated aqueous Na2S2O3, diluted with CH2Cl2 and extracted with CH2Cl2. The organic layer was washed with H2O and brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (EtOAc/hexane, 10:90) to afford a N-BOC aminoalkyliodide. To a solution of NaH (60% in oil, 0.673 g, 16.8 mmol) in DMF (15.3 mL) was added dropwise a solution of N-hydroxyphthalimide (2.49 mmol) in DMF (10 mL) at 0 C under an argon atmosphere. After 15 min of stirring, a solution of the iodide (15.3 mmol) in DMF (10 mL) was added dropwise to the solution and stirred at 70 C for 12 h. After cooled to 0 C, the reaction was quenched with H2O and filtrated to afford colorless solid, which was recrystallized to give a N-alkoxyphthalimide 13a-d. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping